America’s Hidden National DNA Database
Introduction
Within days of birth, nearly all infants born in America are compelled to give their DNA to the government.[1] Hospital staff warm, sterilize, and prick a newborn infant’s heel, collecting a blood sample on a newborn screening card.[2] These newborn blood spots are taken for good reason. Every state operates a public health program that collects and analyzes these blood samples for a wide range of metabolic, genetic, and other disorders.[3] Where a disorder is detected, early intervention can have a profound impact on the child’s development, life expectancy, and quality of life.[4] Newborn screening programs have improved and saved thousands of lives.[5] They are a critical part of our public health infrastructure.[6]
However, after this public health screening is complete, some portion of the blood sample remains.[7] States typically store these residual newborn blood spots for quality assurance, research, or other purposes.[8] Some states store these blood samples for months—but often, states store these blood spots for years or even decades.[9] Recently, some law enforcement investigators have tapped this resource in criminal investigations.[10]
That law enforcement would seek access to this rich trove of genetic material now is not entirely surprising. Since the April 2018 arrest of the Golden State Killer, police have been eager to use nonforensic genetic data in service of forensic goals.[11] In the Golden State Killer case, investigators developed a genetic profile from crime scene evidence, uploaded it to several consumer genetic sites, and compared it with the profiles of millions of ordinary Americans, exploiting connections to distant relatives to home in on a suspect.[12] In the three years since the Golden State Killer’s arrest, investigators have undertaken hundreds of similar investigations.[13]
Having discovered one nontraditional DNA repository in which to develop investigative leads, some police have already set their sights on another. In December 2020, reporters uncovered that investigators in California had sought access to newborn screening samples for criminal investigative purposes.[14] Police had already made at least one arrest stemming from the use of these resources.[15]
This use is poised to grow. Traditionally, state labs engaged in newborn screening have examined molecules in the blood indicating a genetic disorder or isolated segments of genetic material.[16] But some researchers and clinicians have advocated for broader genetic sequencing at birth to “facilitat[e] a lifetime of personalized medical care.”[17] Were such sequencing routine, the data generated from newborn screening would effectively amount to a national DNA database.[18] Even in the absence of routine genome-wide sequencing, more limited sequencing of newborn screening samples could take hold, whether for tracking samples over time, for research use, or even for more straightforward law enforcement purposes.[19]
As law enforcement interest in investigative genetic genealogy demonstrates, police are eager to exploit nontraditional genetic repositories for investigative use. That is so, notwithstanding the expectations of the progenitors of the genetic data those repositories hold.[20]
Whether, and under what circumstances, law enforcement should be able to access residual newborn screening samples or their related data is thus an urgent matter. Scholars have previously focused on the screening, storage, or research uses of newborn screening resources—and matters of informed consent related to each.[21] But this Article is the first to comprehensively survey and map state statutory and regulatory policies governing nonconsensual law enforcement access to these valuable resources.[22]
In so doing, this Article makes three contributions to the existing literature. First, the Article joins a burgeoning scholarship that bridges the bioethics and criminal justice literatures to shed light on genetic resources—and uses of those resources—across domains.[23] For too long, much legal scholarship has treated clinical and research genetic data as distinct from forensic genetic data. This Article focuses on the ways in which the walls separating these types of resources have eroded.
Second, after Part I provides background on newborn screening programs and forensic genetic identification, Part II surfaces the complex constellation of state policies governing law enforcement access to newborn screening samples and related data. Part II demonstrates that more than a quarter of U.S. states have no discernible policy in place regarding law enforcement access, while nearly a third may permit such access in at least some circumstances. Many states’ policies precluding or permitting law enforcement access must be inferred, as these policies fail to identify law enforcement specifically. On the whole, state policies reflect a troubling inattention to this looming issue.
Third, having mapped this disarray of policies, Part III argues that state policymakers should adopt clear policies rejecting law enforcement use of newborn screening resources to develop investigative leads. Such policies are best able to maintain public trust in the public health purposes of newborn screening programs, most consonant with respect for persons, and most certain to survive constitutional scrutiny. Moreover, until such policies are in place, laboratory personnel or courts facing law enforcement requests for access should resist such requests where possible. Newborn screening programs serve the public health. Law enforcement interest must not be permitted to undermine public trust in these programs or to dissuade new parents from participating in them.
I. Situating Genetic Data
This Part briefly introduces newborn screening programs, as well as law enforcement use of genetic data to solve crimes. These programs have existed independently and separately for decades. Subpart I(A) traces the history of newborn screening from the advent of Guthrie cards to the push for genome-wide sequencing. Subpart I(B) traces the separate history of forensic use of DNA for identifying criminal suspects. Subpart I(C) brings these two together, positing that both expanding use of DNA sequencing in newborn screening and growing law enforcement appetite for using non-law-enforcement-derived genetic data to generate leads may bring newborn screening samples and related data within investigative sights.
A. A Primer on Newborn Screening
Newborn screening programs first launched in the 1960s.[24] Today, these programs typically consist of a blood test, hearing test, and screening for congenital heart defects.[25] For the blood test, hospital staff collect several blood samples on special filter paper, commonly known as a Guthrie card.[26]
The first state newborn screening program screened for only one disorder, phenylketonuria (PKU).[27] PKU is an inborn error of metabolism, one of many rare genetic disorders that interferes with the body’s normal metabolism.[28] In a child affected with PKU, the body cannot make a particular enzyme needed to break down an amino acid, phenylalanine, and so phenylalanine instead builds up in the brain.[29] Untreated, PKU invariably results in severe mental disability, seizures, and other neurological problems.[30] But if PKU can be detected in early infancy, then a child may be given a low-phenylalanine diet and instead experience more normal development.[31] By the early 1970s, all states had adopted newborn screening for PKU, with most utilizing centralized, state-run laboratories to conduct the analysis.[32]
States thereafter slowly and haphazardly expanded their newborn screening programs.[33] The scope of newborn screening varied significantly from state to state. As late as 2003, state newborn screening programs examined between four to thirty-six disorders, with most states screening for eight or fewer conditions.[34] But the development of tandem mass spectrometry made expanded screening a practical reality.[35] Traditionally, newborn screening required a separate test for each condition that was a part of the state’s program.[36] Tandem mass spectrometry, by contrast, enabled state laboratories to screen for multiple conditions simultaneously.[37]
Concern about the wide variability of newborn screening programs in different states eventually coalesced into national recommendations. In 2005, the American College of Medical Genetics (ACMG) developed robust criteria for determining when a condition should be added to a newborn screening program and identified twenty-nine conditions satisfying those criteria.[38] These twenty-nine conditions had “a screening test, an efficacious treatment, and adequate knowledge of natural history.”[39]
In 2007, Congress passed the Newborn Screening Saves Lives Act.[40] The Act directed the creation of an Advisory Committee on Heritable Disorders in Newborns and Children, which was tasked with developing a recommended uniform screening panel (RUSP) and criteria for adding to it.[41] That Committee began by “adopting the initial 29 core disorders outlined by ACMG’s report and develop[ing] a nomination and review process for the addition of disorders to the RUSP.”[42] Since 2007, several additional conditions have been added to the RUSP, while other candidate conditions have been rejected.[43] In all, the RUSP now includes thirty-five “core” conditions and twenty-six “secondary” conditions.[44] Although the RUSP does not create a federal mandate, “[t]oday, all states and territories in the United States offer expanded [newborn screening] for the initial panel of conditions, with more recently added conditions slowly being taken up by the individual states.”[45]
Throughout the history of state newborn screening programs, states have given little role to parental consent. Affirmative parental consent for newborn screening is rarely sought.[46] Indeed, “[i]n most states (and in most programs in the developed world) [newborn screening] is mandatory, with few, if any, options for parents to opt-out for their child.”[47] Ordinarily, such an imposition on the traditional right of parents to oversee the upbringing of their children and to make medical decisions on their behalf would be untenable.[48] But proponents have offered two justifications for proceeding with newborn screening programs in this way. Some have argued that parental consent is unnecessary where the state exercises its general police powers to preserve public health.[49] Others have suggested that newborn screening programs can be justified as an exercise of the state’s parens patriae power,[50] which permits the state “to substitute its authority for that of natural parents over their children.”[51]
As newborn screening programs have evolved and grown in their sweep and scope, some scholars have questioned the fit between these justifications and modern newborn screening programs.[52] But even if these justifications suffice to support taking and screening newborns’ blood without parental consent, it is far from clear that those same justifications support the subsequent retention and use of those samples for other purposes.
Yet such retention and secondary use is common. State laboratories routinely hold on to the residual newborn blood spots that remain once the lab has completed the newborn screening tests.[53] State policies vary widely in the length of time for which retention is permitted, and some states do not directly regulate this matter at all.[54] According to a survey published in 2011, as many as eighteen states simply do not address the retention of newborn screening samples in their state newborn screening laws.[55] Among those that do regulate retention, states have varying provisions, with some retaining samples only for weeks, while others permit or require retention for years, decades, or even indefinitely.[56]
Similarly, subsequent use or release of newborn blood spots for purposes other than newborn screening itself is also common. As Sonia Suter has observed, “these blood spots, like most pathology samples, are a treasure trove for researchers because they are a valuable national repository of genetic material.”[57] Yet, state laws regulating such research uses, where they exist at all, often leave something to be desired. As of 2011, only thirteen states specified research purposes to which residual newborn screening samples could be put, and in many instances, these purposes were broadly stated and therefore provided only limited guidance.[58] Even fewer states regulated the secondary uses to which newborn screening data may be put.[59] And in many instances, affirmative parental consent is not sought for this use either.[60]
This latter aspect of newborn screening programs has been the subject of substantial controversy—and concerns about undermining public trust in newborn screening programs more broadly. In a pair of cases in 2009, parents of children born in Minnesota and Texas sued their respective states, arguing that retaining and using newborn screening samples for research and other purposes—without parental knowledge and consent—was unlawful.[61] The Texas case, Beleno,[62] settled, but only after the State agreed to destroy more than five million stored newborn blood spot cards and amended its law to permit parents to request destruction of their children’s residual newborn blood spots.[63] The Minnesota Supreme Court in the Bearder case,[64] meanwhile, vindicated the parents’ claims that the nonconsensual “use, storage, or dissemination” of residual newborn blood spots beyond newborn screening itself ran afoul of Minnesota’s Genetic Privacy Act.[65] Minnesota, like Texas, thereafter destroyed previously collected and stored newborn screening samples.[66] Also like Texas, however, Minnesota subsequently amended its laws to expressly permit the state to retain and store newborn screening samples absent a request for destruction, and to permit research use of these samples so long as broad parental consent is obtained.[67]
For a few years, Congress intervened in the consent-to-research debate involving residual newborn screening samples. In the Newborn Screening Saves Lives Reauthorization Act of 2014, Congress specified that “[r]esearch on newborn dried blood spots shall be considered research carried out on human subjects,” and thus subject to the protections of the Federal Policy for the Protection of Human Subjects (the Common Rule).[68] More specifically, Section 12 of the Act, entitled “informed consent for newborn screening research,” barred waiver of informed-consent requirements for the use of such samples in federally funded research.[69] Under this policy, federally funded researchers were permitted to use recent newborn screening samples only where parents had given informed consent for such research use. But this protection lapsed in 2018, following amendments to the Common Rule.[70] Today, as a result, “many state health departments do not obtain consent to collect and analyze [newborn screening samples], nor to store and use the [newborn screening samples] for further research.”[71]
Importantly, neither older single-disorder tests nor newer mass spectrometry methods analyzed a child’s genetic sequence directly. To be sure, the conditions that were part of a state’s program had a genetic basis.[72] But screening tests largely analyzed the presence, absence, or amount of specific compounds or chemicals in the blood.[73] In other words, these tests looked for the product of a genetic variant, rather than for the variant itself.
But genetic sequencing has by now become a part of newborn screening programs, at least in part. Genetic sequencing first entered newborn screening programs as part of testing for cystic fibrosis.[74] States screening for cystic fibrosis first perform a more traditional test, measuring an enzyme in the blood.[75] If a newborn screening sample contains a high level of this enzyme, many states proceed to sequencing of the gene associated with cystic fibrosis.[76] States utilizing genetic sequencing as a secondary test for cystic fibrosis vary in the depth of sequencing undertaken, with some using “full gene sequencing of affected infants to create a unique, and maximally sensitive, panel for their state.”[77] Nor is cystic fibrosis the only condition screened with genetic sequencing. At least two other conditions, severe combined immunodeficiency and spinal muscular atrophy, are detected using genetic sequencing technology.[78]
More broadly, the use of genetic sequencing technology is poised to grow in the coming years. For one thing, as others have observed, “many [newborn screening] labs already have the capacity for DNA extraction and manipulation.”[79] For another, there is growing interest from clinicians, researchers, and others in developing much broader genetic sequence data for newborns, in the hopes of bringing about the promised personalized-medicine revolution or simply to facilitate broader newborn screening efforts.[80] These proposals have sought to introduce genome-wide sequencing to newborn screening.[81] As recently as 2021, directors of the federal National Human Genome Research Institute asserted that, although “[t]oday’s newborn screening involves analysing metabolites, . . . a broader implementation that includes genome sequencing will eventually happen.”[82] These experts contended that “the central issue is timing.”[83]
Genome-wide sequencing for newborns has already been planned or implemented in some instances. In 2013, the U.S. National Institutes of Health (NIH) initiated a research program involving this kind of newborn genetic sequencing.[84] In late 2019, the United Kingdom went one step further, announcing a pilot project for newborn genome-wide sequencing, with plans to expand the program to all newborns in the future.[85] Although many have criticized the push for genome-wide sequencing,[86] it seems likely that such efforts will persist—and grow.
Even if genome-wide sequencing does not take hold in newborn screening, sequencing of the very genetic markers used for law enforcement purposes might. Authentication, or confirmation of the stable and verifiable identity, of biospecimens has been a persistent issue in the research community. Since 2015, the NIH has required that federally funded researchers authenticate certain biospecimens used in research.[87] For a variety of reasons, both historical and economic, most research authentication today uses many of the same genetic data points that law enforcement uses for crime-detection purposes.[88] Efforts to authenticate newborn screening samples—a potentially worthy goal given both the importance of confirming identity of newborn screening samples before releasing results and the substantial use of newborn screening resources in subsequent research—could inadvertently give rise to law enforcement-usable data.
One lesson from this history is that newborn screening programs have evolved substantially over time, including in ways that take them far afield from their initial intended purpose. Another is that these programs most seriously court controversy when the blood samples and data they generate are put to uses distinct from those for which they were collected, particularly where those uses may be controversial in and of themselves. The push toward generating broader swaths of genetic sequence data as part of newborn screening may make those subsequent uses much broader—and perhaps more controversial, too.
B. A Primer on DNA Forensics
Law enforcement’s analysis and use of genetic data to investigate crimes, like newborn screening efforts, dates back decades. Virginia established the first forensic DNA database in 1989.[89] In 1994, the federal DNA Identification Act directed the FBI to create a national backbone for coordinating forensic DNA databasing across state lines.[90] Pursuant to that mandate, the FBI established the Combined DNA Index System (CODIS), a central database through which participating states and agencies collect, store, and share lawfully obtained genetic profiles.[91] Today, all fifty states, the District of Columbia, Puerto Rico, and the federal government participate in CODIS.[92]
CODIS, and forensic genetic identification more broadly, depend on the fact that genetic data is a durable and individualized identifier. Unlike a credit card number, an individual’s genetic information is immutable.[93] Moreover, genetic identification relies on the fact that individuals are nearly all genetically distinct in small but significant ways.[94] Although all human beings are more than ninety-nine percent genetically identical, even hundredths of a percent represent a very large number of individual genetic differences.[95] “CODIS profiles consist of forty data points drawn from twenty highly variable locations” across noncoding portions of the human chromosomes.[96]
The range of individuals whose DNA is stored in CODIS has expanded dramatically since its earliest days. Many of the early DNA collection statutes limited their reach only to convicted sex offenders.[97] Today, by contrast, nearly all states and the federal government collect and retain DNA from all individuals convicted of a felony and most states compel DNA collection from individuals convicted of some misdemeanors.[98] Additionally, more than half of states and the federal government authorize DNA sampling of individuals arrested for, but not yet convicted of, some crimes.[99] According to the FBI, as of October 2021, CODIS contained nearly fifteen million “offender” profiles and an additional 4.5 million profiles from arrestees.[100]
Despite this growth in size and scope, certain limitations have remained—at least as far as CODIS is concerned. To date, no jurisdiction has authorized, or even seriously entertained, the collection and retention of DNA from ordinary members of the public for crime-detection purposes.[101] When states have proposed to collect and include in CODIS the genetic profiles of individuals not associated with the criminal justice process, public outcry has swiftly followed.[102] Moreover, genetic profiles from volunteers are typically ineligible for inclusion in CODIS.[103]
Recent innovations in the field of forensic genetic identification, however, have altered the landscape and whet the appetite of law enforcement for access to genetic data from new sources. In April 2018, police arrested Joseph James DeAngelo, charging that he was the Golden State Killer, responsible for more than a dozen murders and fifty sexual assaults throughout the 1970s and 1980s.[104] After decades of dead ends, police finally identified DeAngelo as the Golden State Killer by comparing a DNA profile derived from crime scene evidence to other DNA profiles searchable in online consumer genealogical databases.[105] Those searches uncovered some distant cousins of the Golden State Killer.[106] By sleuthing in that extensive family tree, investigators ultimately homed in on DeAngelo and arrested him to great acclaim.[107] This investigative method, comparing crime scene DNA to consumer genetic profiles, is called “investigative genetic genealogy” or “forensic genetic genealogy,” acknowledging its law enforcement purpose and both its genetic and genealogical methods.[108]
Investigative genetic genealogy searches are importantly different from those conducted in CODIS. As described above, to date, no state (or other CODIS-participating agency) has permitted DNA profiles to be stored for routine crime-detection purposes from individuals who lack a legitimate law enforcement connection.[109] Consumer-genetics platforms, meanwhile, are composed of genetic data from millions of individuals with no known law enforcement connection.[110] Indeed, law enforcement entities have argued that accessing and using these data is lawful precisely because users of consumer-genetics platforms have “volunteered” their data,[111] a justification that would not support use of their data through CODIS.
Genetic data housed on consumer-genetic platforms are also much more intrusive than CODIS-related genetic data. CODIS utilizes forty genetic data points in noncoding DNA, and its genetic profiles were designed to be “maximally informative about individual identity, but minimally informative about anything else.”[112] Genealogical DNA profiles, by contrast, consist of hundreds of thousands of DNA data points, strewn across both coding and noncoding DNA, and designed to give rise to a host of interesting and actionable genetic insights.[113] As the Golden State Killer investigation itself demonstrates, these data can reveal second, third, or even more distant cousins.[114] Moreover, such data may reveal an individual’s health risks, physical traits, or other potentially sensitive information.[115]
Finally, investigative genetic genealogy exploits the sprawling family trees that consumer genetic data make possible to reach nearly national coverage for forensic genetic identification (at least for individuals of European ancestry). Genetic data housed in CODIS are largely used to identify the individuals whose cells were used to produce the genetic profiles stored there. Moreover, in several states, investigators may seek to identify partial matches between a crime scene profile and known offenders, which might indicate that a genetic relative of a known offender committed the crime in question.[116] But these familial identifications are largely limited to first-order relationships, like parent–child or full genetic siblings.[117] With access to only a few million consumer-genetics profiles, by contrast, investigative genetic genealogy is likely capable of identifying virtually any American of European descent.[118]
Perhaps unsurprisingly, with the arrest of the Golden State Killer so amply demonstrating the power of investigative genetic genealogy, law enforcement efforts to utilize this technique have rapidly materialized and continue to grow. Parabon NanoLabs claims that it has already identified at least two hundred suspects this way.[119]
But investigative genetic genealogy can be costly and not necessarily straightforward. Utilizing this new technique for forensic genetic identification typically requires that law enforcement work with a private, outside company to prepare the appropriate genetic profile from crime scene evidence. Local law enforcement is also typically ill-equipped to complete the genealogical investigation that connects the crime scene DNA profile to a known, but distant, genetic relative. These services can run to thousands of dollars per case.[120] Consumer-genetics platforms that work with law enforcement may charge yet another fee for the privilege of using their site for investigative purposes.[121] On top of these expenses, investigative genetic genealogy may be difficult to complete. High-quality genealogical research of the sort required for successful investigative genetic genealogy demands expertise, as it may require constructing a family tree spanning several generations both back into history and forward through time.[122] Finally, there may be quality-assurance-related concerns regarding both the scientific and genealogical components of an investigative genetic genealogy search. On the scientific front, there are no national regulatory requirements for data quality or assurance for laboratories engaged in consumer genetic services.[123] As for genealogy, there are no professional standards genetic genealogists must meet before working with law enforcement.[124]
These concerns may dissipate as investigative genetic genealogy becomes more established and consumer-genetics platforms continue to grow in size.[125] But they indicate that law enforcement may also be eager to find more straightforward, alternative methods for reaching a broad population for forensic identification.
C. Newborn Screening as DNA Forensics
The notion of using newborn screening resources for forensic genetic identification is not wholly new. Articles discussing newborn screening have identified such use as a potential risk.[126] Scholars who have suggested expanding CODIS, or a similar effort, on a comprehensive and national scale likewise have explained that newborn screening programs would make this easier to implement.[127]
But the advent and rapid growth of investigative genetic genealogy has heightened the probability that such use may be forthcoming. Investigative genetic genealogy breaks the link between government custody and routine genetic surveillance. It repudiates the principle, central to many judicial decisions approving the retention and use of genetic profiles in CODIS, that only noncoding DNA will be used.[128] And it makes routine genetic surveillance commonplace even for ordinary, law-abiding Americans.[129] Investigative use of newborn screening samples or related data would double down on many of these features of investigative genetic genealogy.
Moreover, investigative use of newborn screening resources may be more attractive to law enforcement than even investigative genetic genealogy. Newborn screening relies on samples that are collected by trained staff and analyzed in laboratories that comply with thorough quality assurance standards.[130] Newborn screening draws on samples and data already in government hands.[131] And these resources are more genuinely comprehensive in scope, unlike investigative genetic genealogy, which is comprehensive only through deducing often distant genetic relationships. These features could make investigative use of newborn screening resources more cost effective than investigative genetic genealogy. Among other advantages, using newborn screening resources would eliminate at least two of the expenses associated with investigative genetic genealogy, namely payments to consumer-genetics platforms for access and payments to highly skilled genealogists. With a comprehensive database, on the state (or possibly national) level, little or no genealogy would be required to make an identification.
Current features of newborn screening programs might pose a logistical roadblock to straightforward use of such resources for investigative purposes—for now. As described above, existing newborn screening tests tend to rely on indirect indicators of a genetic condition, rather than on genetic sequencing directly.[132] But genetic sequencing has been growing in use in newborn screening, and efforts to shift newborn screening to genome-wide sequencing presage a more data-rich future.[133] Even in the absence of genome-wide sequencing at birth, moreover, newborn screening samples might be used in a variety of ways to generate genetic data that law enforcement might wish to exploit. State labs, researchers, or others with access to these samples might, for instance, conclude that it is productive to sequence the CODIS loci for newborn screening samples, whether for authentication, research, or more straightforward law enforcement purposes.[134] More broadly, the mere collation and retention of newborn screening samples on a wide scale might invite law enforcement interest in extracting CODIS or other genetic sequence data from those samples in the first instance.[135]
With investigative genetic genealogy having opened the eyes of law enforcement to the wealth of genetic data housed in other repositories, it is likely only a matter of time before there are investigative efforts to tap the potential of the most comprehensive set of identifiable genetic samples. Indeed, law enforcement investigators have sought access to newborn screening resources in at least one case already. In December 2020, reporters in California disclosed that law enforcement in that state had accessed newborn screening samples for criminal investigative purposes, culminating in at least one arrest.[136] This is unlikely to be the last case of its type.
Yet little has been known about what policies states have in place for mediating law enforcement access to this powerful resource. It is to these policies that this Article now turns.
II. Mapping the Regulatory Landscape
In 2003, the federal government canvassed state policies governing newborn screening.[137] That report observed that, of twenty-five states with general genetic privacy statutes, twenty-three nonetheless permitted some disclosure of genetic data without consent.[138] Of those twenty-three, seventeen permitted disclosure under the general genetic privacy statute “[i]n connection with law enforcement or legal proceedings.”[139]
But this analysis leaves much to be desired by a modern audience. The 2003 GAO Report consisted merely of an on–off tabulation of general genetic privacy statutes.[140] This level of analysis (or lack thereof) fails to capture the genuine array of policies that states have now adopted. Moreover, the legal and scientific environments in which both newborn screening and forensic genetic identification now take place are significantly different from those of 2003. The GAO’s 2003 Report predates the development and standardization of the recommended uniform screening panel for newborn screening programs.[141] The GAO Report also predates more recent litigation regarding state retention and secondary use of newborn screening samples without parental consent, efforts to pilot genome-wide newborn screening, and the arrival of investigative genetic genealogy on the law enforcement scene.
Taken together, the time is past due for a close and comprehensive assessment of state policies that may regulate law enforcement access to newborn screening samples and related data. Subpart II(A) briefly describes the methodologies employed in conducting this survey. Subpart II(B) reports the survey results.
A. Survey Methodology
This survey included review of statutory, regulatory, and other sources for each of fifty-one jurisdictions, encompassing each state and the District of Columbia. For each jurisdiction, research began with the LawSeq database, a “searchable database[] of relevant federal and state law and secondary sources to provide an initial portal into the state of genomics law.”[142] Where LawSeq identified relevant legal sources, researchers confirmed these sources on Westlaw and proceeded from there. Where LawSeq failed to identify a relevant legal source, researchers turned to jurisdiction-restricted searches in Westlaw, using search terms including “newborn screening,” “newborn blood spot data,” “newborn data,” “genetic data,” and “genetic screening.”[143] Results were screened under “Statutes and Court Rules” and “Regulations.” Upon identifying some relevant material about a jurisdiction’s newborn screening program, researchers reviewed all related sections in the relevant title or chapter, and relevant cross-references.
Finally, researchers queried Google for a jurisdiction’s newborn screening program, which would often identify the state health department’s website. These public websites sometimes disclosed relevant information regarding secondary use of newborn screening samples or related data. Where information disclosed on such websites was subsequently located in state statutory or regulatory documents, it is reflected in the data reported below. But where such statements could not be independently verified in an official source, and therefore appeared to be statements without the backing of law, they were not included.
Initially, multiple researchers investigated each jurisdiction, before comparing results. This effort ensured that the research returned complete results and confirmed that each researcher could work independently to identify the relevant legal sources. Once each researcher demonstrated an ability to identify and compile an accurate and complete result, further jurisdictions were investigated by one research assistant, with review by the author.
B. Survey Results: Policies Vary Widely
In all, data was gathered for fifty-one jurisdictions, including the District of Columbia. But this does not mean that all fifty-one jurisdictions have articulated a policy governing law enforcement access to both newborn screening samples and related data. As discussed below, many states have articulated a policy for newborn screening samples or related data, but not both.
Moreover, more than one-quarter of states appear to have no clearly articulated policy governing law enforcement access to these valuable public health resources at all. Figure 1 identifies those states that lack any discernable policy governing law enforcement access to newborn screening samples and related data, as compared with the majority of states that have some policy in place for one or both of these resources.
Figure 1. Rules and Their Absence
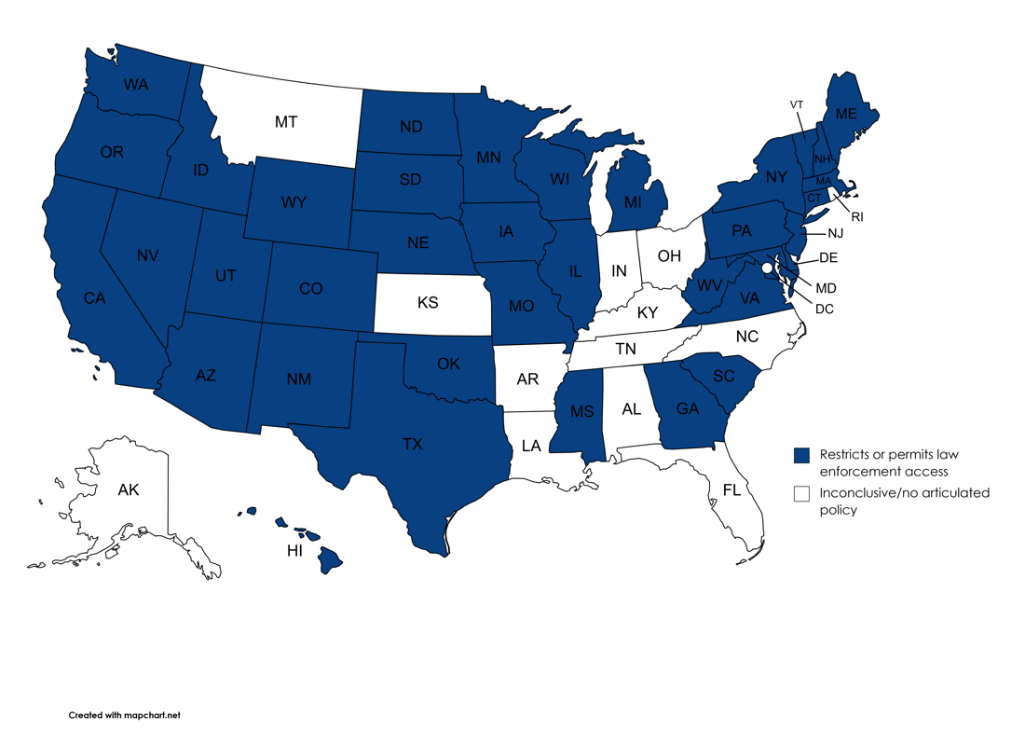
| Policy Type | Number of Jurisdictions |
| Permits or restricts some law enforcement access | 37 |
| Inconclusive/no articulated policy | 14 |
In some instances, a lack of guidance on law enforcement access in this context reflects a broader lack of guidance as to the retention or subsequent use of, or access to, these newborn resources. For instance, neither Rhode Island’s statutes nor its regulations governing its newborn screening program make any mention of the retention or subsequent use of newborn screening samples or related data, much less law enforcement’s access to these resources.[144] Kentucky law is similarly vague. There, the state statute authorizing the newborn screening program instructs that the secretary of the Kentucky Cabinet for Health and Family Services shall promulgate regulations prescribing “the manner of procedures, testing specimens, and recording and reporting the results of newborn screening tests.”[145] But the regulations pursuant to the statute do not give guidance on the recording of data from newborn screening, nor about the confidentiality of or access to the newborn screening samples or related data.[146] Where a state has wholly failed to address issues of retention, use, and access, it is unsurprising that law enforcement access and use has similarly escaped attention.
In other instances, states may have declined to address subsequent access to or use of newborn screening resources due to other provisions preventing those resources’ long-term retention. In Tennessee, for instance, both the sample and “form containing the identifying information” about a sample “shall be destroyed” after one year.[147] Montana similarly requires that “[a]ny dried blood specimens sent to a laboratory approved by the department for [newborn] testing . . . be destroyed after one year by the approved laboratory.”[148] The prospect of law enforcement use may be sharply diminished where newborn screening resources are promptly destroyed. In effect, these states have embraced practical, rather than legal, constraints on subsequent use of newborn screening resources, including by law enforcement.
Even among states with some discernable policy on the books, these policies range in their substance, coverage, and specificity. At a high level of generality, nearly a third of states likely permit law enforcement access to either newborn screening samples or data, while the remainder do not. Figure 2 below maps state policies according to law enforcement access.
Figure 2. Mapping State Policies
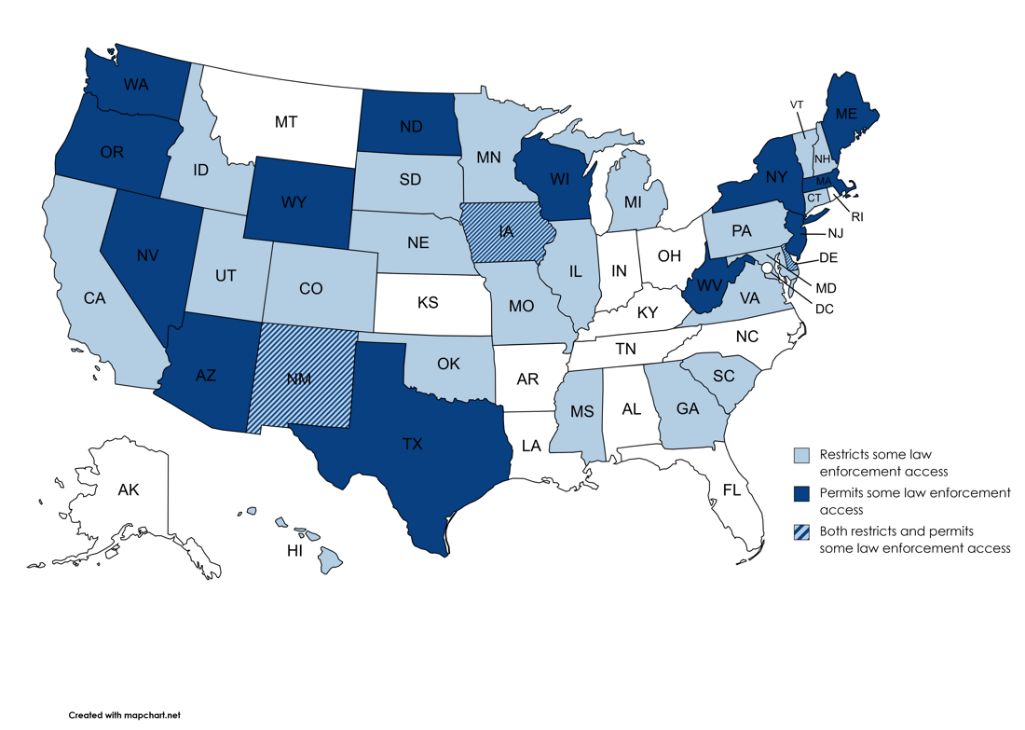
| Policy Type | Number of Jurisdictions |
| Restricts some law enforcement access | 21 |
| Permits some law enforcement access | 13 |
| Both restricts and permits some law enforcement access | 3 |
| Inconclusive/no articulated policy | 14 |
The sections that follow tease apart the varying policy approaches, from clearly articulated prohibition, to clearly articulated permission, to degrees of inferentially determined policies in between. As Figure 2 indicates, and section II(B)(4) discusses, a few states that restrict law enforcement access to newborn screening samples nonetheless have provisions that may facilitate access to the related data.[149]
The overwhelming thrust of these data is that most states have not grappled sufficiently—or at all—with the question of law enforcement access to newborn screening resources. This is true even for states that may have some discernible policy in place. For instance, as discussed below, many of the states that permit some form of law enforcement access appear to have done so have inadvertently. Laboratory personnel facing requests for law enforcement access, state legislators and regulators contemplating change, and courts adjudicating law enforcement submissions should be loath to interpret these provisions broadly, much less as the genuine will of the people.
More broadly, choice of policy does not map neatly onto a red-state/blue-state divide. Among consistently Republican-aligned states, North Dakota’s law provides that “[i]nformation and testing materials received or generated by the newborn screening program . . . are confidential except as provided by law or regulation,” an exception of indeterminate breadth.[150] Other states are more restrictive. Mississippi’s law is short and clear: “Under no circumstances will the retained specimen be used for research or purposes other than confirmation of previous test results.”[151] South Dakota similarly provides, “Upon completion of newborn screening testing, the designated laboratory is responsible for specimen destruction in a secure manner. No specimen may be used for any purpose other than the screening of newborn infants . . . .”[152]
Similar divides in policy are evident among consistently Democratic-leaning states. Washington’s administrative code authorizes disclosure of newborn screening data upon a court order, as well as “when required by state or federal law.”[153] But Connecticut and Hawaii, also Democratic strongholds, take a different view. Hawaii’s law provides, “[a]ll information, including records, correspondence, and documents, specific to individual newborns, shall be confidential and shall be used solely for the purposes of medical intervention, counseling, scientific research, or reporting.”[154] Connecticut’s statutory scheme is even more precise, announcing that such data “shall not be admissible as evidence in any action of any kind in any court or before any other tribunal, board, agency or person, nor shall it be exhibited or its contents disclosed in any way, in whole or in part.”[155]
1. Clear Policies Barring Access.—
Restrictive standards on access are frequently embedded directly and clearly in newborn screening policies. Iowa has the clearest and most specific prohibition of law enforcement access, at least with respect to newborn screening samples. In a subsection entitled “[p]rohibited uses,” the state unequivocally declares, “[a] residual newborn screening specimen shall not be released to any person or entity for commercial purposes or law enforcement purposes or to establish a database for forensic identification.”[156] It is difficult to imagine a more direct or clear prohibition on law enforcement access to newborn screening samples.
Other states with clear policies restricting access do not mention law enforcement by name. Connecticut’s statutory scheme, described above, is one such example, which broadly precludes the disclosure or use of relevant data in court proceedings.[157] Illinois adopts a similar constraint in its more general genetic privacy statute. That statute explains that “[a]ll information and records held by a State agency, local health authority, or health oversight agency pertaining to genetic information shall be strictly confidential,” and “shall not be admissible as evidence nor discoverable in any action of any kind in any court or before any tribunal, board, agency, or person.”[158] Lest there be confusion about whether newborn screening data is governed by this section, the statute carves out from its coverage disclosures “made for the sole purpose of implementing the Newborn Metabolic Screening Act and rules.”[159] This carve-out makes clear both that newborn screening data is relevant genetic information and that uses of that data beyond straightforward implementation of the screening program itself are protected under the more general statute.
Other regulatory regimes take yet another approach, explicitly restricting access to specified purposes and thereby rendering all unnamed purposes beyond the bounds of the law.[160] Minnesota offers the most detailed statutory scheme of this type. In 2011, the Minnesota Supreme Court held that both newborn screening samples and related data are “genetic information” subject to the protections of the state’s genetic privacy statute.[161] In response, the state legislature amended its newborn screening statute to align with the court’s opinion.[162] The revised statute permits parents separately to refuse newborn screening itself, the retention of residual newborn screening samples and related data for “program operations,” or the dissemination of those resources for research purposes.[163] The statute goes on to define “program operations” as “actions, testing, and procedures directly related to the operation of the newborn screening program, limited to the following,” followed by a list of relevant activities, none of which include law enforcement or criminal investigation.[164] By explicitly limiting the scope of program operations to identified activities, Minnesota effectively precludes other activities, including law enforcement ones.
New Hampshire also treats newborn screening samples and related data similarly, extending equally limited access to both. But it does so with far fewer words: “Residual [newborn screening] specimens and related records may be retrieved for other purposes only with the written authorization of a parent or guardian.”[165]
Idaho strictly limits the use only of newborn screening samples. Its regulations first set out permitted uses of these samples, stating that they “will be used for the purpose of testing the infant from whom the specimen was taken, for congenital birth defects. Limited use of specimens for routine calibration of newborn screening laboratory equipment and quality assurance is permissible.”[166] The same section then elaborates complementary “[p]rohibited use[s],” stating, “[newborn screening] specimens may not be used for any purpose other than those described in [the preceding section] of this rule without the express written consent of the parent(s) or guardian(s) of the infant from whom the specimen was collected.”[167]
Vermont is similar. Its regulations state that “[newborn] bloodspots may be used without parental consent by the testing laboratory only for the purpose of quality assurance and quality control for routine maintenance and function checks.”[168] The regulations then reinforce that limitation, stating separately that “[newborn] bloodspots shall not be used for any other purposes without written consent from a parent or guardian.”[169]
Mississippi brooks no confusion, stating bluntly, “Under no circumstances will the retained specimen be used for research or purposes other than confirmation of previous test results.”[170] South Dakota similarly provides, “No specimen may be used for any purpose other than the screening of newborn infants pursuant to [the state newborn screening statute].”[171]
Colorado and Pennsylvania adopt yet another variation on this theme. Colorado, having described “all information gathered” through the newborn screening program as “confidential,” specifies that “[p]ublic and private access to newborn patient data is limited to data compiled without the newborn’s name.”[172] Pennsylvania, in its section on “confidentiality,” states that persons and entities involved in the newborn screening program “may not release any identifying information relating to any newborn child screened in the newborn screening program” outside of that program’s participants “except” with consent or as necessary to provide services to an affected child.[173]
Finally, several states invoke confidentiality in combination with restrictive language governing permitted disclosures, thus effectively precluding disclosure for nonenumerated purposes. Both Connecticut and Hawaii utilize the word “solely” in defining acceptable uses of newborn screening data.[174] Delaware’s regulations similarly state that newborn screening samples “will only be used for activities to improve the screening program and/or develop new screening tests.”[175] In Maryland, newborn screening information “is confidential and may only be used or disclosed” for four specified purposes, none of which relates to law enforcement use.[176] Under Georgia law, “[i]nformation derived from genetic testing shall be confidential and privileged and may be released only to the individual tested and to persons specifically authorized by such individual to receive the information.”[177] Oklahoma specifies that newborn screening data is “confidential” and “may only be used or disclosed” for specified purposes, none of which is related to law enforcement or criminal investigation.[178] Virginia law is much the same.[179] In pairing an assertion of confidentiality with the restrictive “solely” or “only,” these states mark law enforcement’s exclusion.
Many states have thus enacted, in statute or by regulation, clear prohibitions on uses of newborn screening samples or related data beyond those expressly permitted—including law enforcement use. Explicit prohibitions are possible, though by no means universal.
2. Clear Policies Permitting Access.—
Unlike state policies restricting law enforcement access, no state expressly codifies permission for law enforcement access within the statutes or regulations governing the newborn screening program itself. Instead, states that most clearly appear to authorize law enforcement access do so through more general statutes governing genetic privacy. Nevada law expressly contemplates that “genetic information” may be obtained, retained, and disclosed to assist a criminal investigation.[180] Most relevant for newborn screening data, genetic information may be disclosed without consent “[t]o conduct a criminal investigation, an investigation concerning the death of a person or a criminal or juvenile proceeding.”[181] The Nevada statute makes clear that newborn screening data is considered “genetic information,” permitting such information to be obtained and disclosed without consent “[t]o determine the presence of certain preventable or inheritable disorders in an infant pursuant to” the state’s newborn screening program.[182]
New Jersey’s “Genetic Privacy Act” is similar, with nearly identical exceptions, including for nonconsensual disclosure of genetic information where “necessary for the purposes of a criminal or death investigation or a criminal or juvenile proceeding.”[183] As in Nevada, New Jersey also permits nonconsensual disclosures “pursuant to newborn screening requirements established by State or federal law,” making clear both that the newborn screening program produces “genetic information” and that such information is not otherwise protected from disclosure.[184] Moreover, the Genetic Privacy Act expressly contemplates that the law enforcement activities excluded from the Act will go beyond the use of genetic information in CODIS. The Act separately and additionally excludes from its privacy protections disclosures “made pursuant to the provisions of the ‘DNA Database and Databank Act of 1994,’” which governs New Jersey’s participation in CODIS.[185]
In the absence of newborn screening-specific policies related to law enforcement access, these more general provisions are likely to control. The result is that law enforcement would likely be able to access newborn screening data on the same terms that apply to other sources of genetic data.[186] As set out in Part III below, however, there may be good reason to treat newborn screening resources differently than other sources of genetic data.[187] Accordingly, policymakers and adjudicators should be cautious about relying on these statutory sources to draw conclusions with respect to law enforcement access to newborn screening resources.
Iowa perhaps comes closest to directly authorizing law enforcement access through its newborn screening provisions. Iowa’s administrative code provides that “[r]eports, records, and other information collected by or provided to the Iowa newborn screening program” may be released to “[a] representative of a state or federal agency, to the extent that the information is necessary to perform a legally authorized function of that agency or the department.”[188] Although this provision does not identify law enforcement specifically, law enforcement investigators, as representatives of a state or federal agency, might invoke it in seeking to access newborn screening data. But it is far from clear that law enforcement is among those whom Iowa regulators had in mind when they promulgated this regulation.[189] The juxtaposition of this provision with the law enforcement-specific prohibition governing newborn screening samples suggests that the access-to-data provision should be construed narrowly. Moreover, principles of constitutional avoidance may favor a narrower construction of this provision.[190]
3. Policies by Inference.—
In many instances, state policies are less clearly specified than those discussed above. Rather, they must be inferred or cobbled together. Inference may come through statutory or regulatory cross-references, vague exceptions to otherwise strong confidentiality provisions, or merely what appears to be inattention to the issue of law enforcement access to newborn screening resources. Moreover, inference may be necessary to identify either law enforcement exclusion or access. This section identifies representative examples of each.
a. Inferring Law Enforcement Exclusion.—
Several states employ statutory or regulatory frameworks that limit access to newborn screening resources but may not use explicitly restrictive language. Michigan law, for instance, permits newborn “blood specimens to be used for medical research during the retention period . . . as long as the medical research is conducted in a manner that preserves the confidentiality of the test subjects and is consistent to protect human subjects from research risks.”[191] The statutory provisions do not mention any other secondary uses for these samples.[192] Nebraska law similarly provides that newborn screening samples “may be used for public health research, further patient diagnostic testing, and public health purposes, for example, but not limited to, quality assurance and improvement of newborn screening practices.”[193] This list of permissible uses does not include law enforcement investigative purposes, and it is clearly targeted at permitting a limited set of health-related activities. Moreover, although the list of permissible uses explicitly contemplates that other purposes may be pursued, they must be “public health purposes.”[194]
South Carolina’s regulations regarding newborn screening also identify limited purposes for which newborn screening samples may be stored and disseminated: “The Laboratory . . . may release specimens for purposes of confidential, anonymous scientific study unless prohibited by the parents, legal guardians, or children from whom the specimens were obtained when the children are eighteen years of age or older.”[195] The regulations provide the text to be used in seeking parental authorization for sample storage, which goes further, stating, “A child’s blood sample can only be released for approved research, without any identifying information, to learn new information about diseases.”[196] As discussed in subpart II(B)(1), the use of the word “only” should be interpreted to preclude other uses. But as this word appears in the sample form, rather than in the regulatory text itself, its effect may be more inferential than explicit.
In each of these states, the regulatory regime does not contemplate that law enforcement will have access to newborn screening samples. Each state identifies the purposes for which such samples may be released, and they do not include law enforcement or anything similar. The lack of explicit language of exclusivity—like the “only” that appears in other states’ policies—may make law enforcement preclusion somewhat more inferential. But the inference is only slight, and it should not undermine the conclusion that law enforcement access is precluded.[197]
Utah’s regulations largely take a similar approach with respect to newborn screening samples. The regulations provide that those samples will be used or released for quality assurance or approved research purposes.[198] This provision does not include explicitly restrictive language, but it also does not appear to contemplate additional uses.
Utah goes further with respect to newborn screening data, though even this policy demands some legwork to piece together. The relevant regulation states generally that “[a] testing laboratory that analyzes newborn screening samples for the Department may not release information or samples without the Department’s express written direction.”[199] The regulation then gives guidance about how the department must evaluate requests for access to newborn screening data: “All requests for test results or records are governed by Utah Code Title 26, Chapter 3.”[200] The cross-referenced chapter, in turn, makes clear that newborn screening data may not be released to law enforcement. In a section entitled, “Health Data Not Subject to Subpoena or Compulsory Process—Exception,” the Utah statutory code states, in relevant part, “[i]dentifiable health data obtained in the course of activities undertaken or supported under this chapter may not be subject to discovery, subpoena, or similar compulsory process in any civil or criminal, judicial, administrative, or legislative proceeding.”[201] This provision ought to be interpreted as barring law enforcement access to newborn screening data, whether by request, subpoena, or warrant, including for advancing criminal proceedings. But given the circuitous cross-references required to unearth this prohibition, and in contrast with the more direct prohibitions identified in section II(B)(1), supra, it is more a policy by inference.
Missouri’s statutory scheme should also be interpreted to bar law enforcement use of newborn screening resources. Missouri’s newborn screening statute provides that, at most, “a biological specimen may be released for purposes of anonymous scientific study.”[202] The corresponding provision for newborn screening data states that this data “shall be held confidential and be considered a confidential medical record, except for such information as the individual, parent or guardian consents to be released . . . .”[203] As discussed below, invocations of “confidentiality” or a “confidential medical record” are not, standing alone, conclusive as to the law enforcement inquiry. But Missouri’s statutory scheme leaves no doubt that law enforcement is not to have access to this data. In another program under the same chapter of the state code, the confidentiality provisions look much different.[204] Those provisions explicitly provide for disclosure to law enforcement personnel and delineate a process for obtaining a court order for disclosure of test results.[205] Missouri, in other words, knows how to specify and facilitate law enforcement access where appropriate. It did not do so with respect to newborn screening resources.[206]
Finally, California’s regulatory regime appears to preclude law enforcement use of newborn screening samples and related data. California regulations state, “The blood specimen and information obtained during the testing process becomes the property of the State and may be used for program evaluation or research by the Department or Department-approved scientific researchers without identifying the person or persons from whom these results were obtained.”[207] As in other states discussed here, this regulation should be interpreted to permit use of California newborn screening samples only for the identified purposes. As for newborn screening data, California regulations state that this information “shall be confidential and shall be used solely for the purposes of medical intervention, counseling, or specific research project approved by the Department.”[208] Although a subsequent subsection permits disclosures “as provided by law,”[209] these disclosures are likely to be of little value, since the data may only be “disclosed” but not “used” in this fashion. Considering these protections, law enforcement’s already-established use of newborn screening resources for investigative purposes may well have been unlawful—and it stands as a sharp reminder of the costs of inattention to these issues.[210]
b. Inferring Law Enforcement Access.—
For several states, policies likely permitting law enforcement access to newborn screening samples or related data are not expressly stated in either the newborn screening program itself or exceptions to genetic privacy protections more broadly. Rather, such policies could only be inferred, as where state law permits relevant material to be disclosed upon subpoena or court order. For instance, Washington’s administrative code states that both “[d]ried blood spot samples and specimen information may only be released” to “[a] named person in a legally executed subpoena following review and approval of the state attorney general” or to “[a] person to whom release is mandated by order of a court of competent jurisdiction.”[211] Massachusetts, Oregon, and Texas, among others, similarly provide that newborn screening samples, related data, or both may be disclosed in response to a court order.[212] New York enshrines an exception in its statute governing genetic data, permitting nonconsensual disclosure of such data “as provided in an order of a court of competent jurisdiction.”[213]
Wisconsin’s statutory scheme requires more work to parse. Wisconsin law announces a generally broad protection for newborn screening data, stating that “no information obtained” through screening “may be disclosed except for use in statistical data compiled by the department without reference to the identity of any individual and except as provided in s. 146.82(2).”[214] But the cross-referenced code section, as in other states, permits nonconsensual disclosure of this data “[u]nder a lawful order of a court of record.”[215]
In each of these states, disclosure to law enforcement is not specifically contemplated. But law enforcement access may flow from the statutory or regulatory regime. Law enforcement investigators have already demonstrated willingness to seek subpoenas and warrants to gain access to genetic databases compiled for non-law-enforcement purposes.[216]
Wisconsin’s statutory scheme provides further grist for the interpretive mill, though with a different outcome, when it comes to less formal law enforcement “requests” for access. In addition to authorizing data disclosure in response to a court order, Wisconsin further provides that this data may be disclosed “[i]n response to a written request by any federal or state governmental agency to perform a legally authorized function, including but not limited to management audits, financial audits, program monitoring and evaluation, facility licensure or certification or individual licensure or certification.”[217] The reach of “federal or state governmental agency” could include law enforcement entities, who perform a legally authorized function in investigating crimes. But the remainder of this statutory clause suggests that law enforcement may not, in fact, be within its ambit. Law enforcement investigations are dissimilar to each of the government functions named as examples. Although the statute does not confine its reach only to those examples, ordinary principles of statutory interpretation suggest that the government functions for which disclosure is authorized must be similar in kind to those already identified.[218] As law enforcement investigations are not similar in kind to the enumerated functions, disclosure to law enforcement ought not be permissible pursuant to this statutory provision.
Finally, there is a group of statutory and regulatory provisions that seemingly grant law enforcement access to newborn screening resources but whose actual scope and permissiveness is murky at best. Several states permit access or disclosure where otherwise “required by law.” Confusingly, most of these same states broadly assert that newborn screening resources are “confidential.”[219] Washington’s administrative code, in addition to authorizing access upon a court order, provides both that newborn screening resources are “confidential” and that they may “be released when required by state or federal law.”[220] North Dakota and West Virginia similarly require that newborn screening data be disclosed “as provided by law or regulation”[221] or “[a]s required by law.”[222] Meanwhile, Maine’s regulatory scheme permits newborn screening resources to “be used in compliance with confidentiality laws.”[223]
These catch-all exceptions could provide a basis for law enforcement, state laboratories storing samples, or others to conclude that law enforcement access is permissible so long as some statute somewhere permits law enforcement to access some tangentially related data. One such statute might be the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which permits law enforcement to access otherwise confidential, sensitive medical data.[224] Arizona makes this explicit in its genetic privacy statute, which permits “genetic testing and information derived from genetic testing” to be “released only as authorized by state or federal law, including the health insurance portability and accountability act privacy standards.”[225]
But it is not clear whether newborn screening resources, when held by a state laboratory, are actually covered by HIPAA at all, much less its capacious exception for law enforcement access. HIPAA does not protect all medically relevant information.[226] Rather, it only applies to certain “protected health information” held by a “covered entity” or that entity’s business associates.[227] A “covered entity,” in turn, is limited to a health plan, health care clearinghouse, health care provider, and those entities’ business associates.[228] A state public health laboratory or agency does not neatly fit any of these terms.
This murkiness surrounding HIPAA’s application to newborn screening resources suggests that concessions to disclosure as provided, required, or authorized “by law” ought not be read as reflexively permitting broad law enforcement access to newborn screening resources. Rather, for the reasons set forth in Part III infra, and for reasons of clear notice, such provisions should instead be construed narrowly. Perhaps most troubling, the scope of these permitted disclosures is not evident on the face of these regulatory regimes, and both policymakers and the public may mistake this relative silence for protection.
4. Regulating Samples and Regulating Data.—
As the foregoing discussion demonstrates, state regulatory frameworks vary widely, with some states seemingly leaving the option of law enforcement access open due to inattention.
But even this fails to capture the messiness of the regulatory map. Some states have a policy in place for samples, but not necessarily for related data. Other states are the other way around, with a policy in place for data, but not necessarily for the samples themselves. Still other states have clearly articulated policies for both samples and data, but the answer to the law-enforcement-access question differs depending on which resource is at issue. Each of these approaches is discussed in more detail below.
The result of such widely diverging regulatory protections, even within single states, is that it is extremely difficult to accurately and meaningfully capture state approaches to mediating law enforcement access to newborn screening resources. Instead, this question can only be answered haltingly, in fits and starts, and only for pieces of the newborn screening puzzle at a time.
a. Policies for Samples, but Not Data.—
Some states have a policy in place for samples, but not necessarily for related data. Consider Idaho. As described supra in section II(B)(1), that state’s administrative code includes a detailed regulation governing “use and storage of dried blood specimens,” which states that newborn screening samples may not be used for purposes other than the initial screening and limited quality assurance practices unless the parents give consent.[229] But the state regulatory apparatus says nothing about the data generated through newborn screening.
Other states are similar. South Dakota and Vermont, for instance, have strong and explicit provisions limiting how newborn screening samples may be used.[230] But neither state devotes similar attention (or really, any attention at all) to newborn screening data. Mississippi’s newborn screening regulations are particularly interesting in this respect. In a section entitled “Specimen Retention,” the state explicitly bars any nonscreening use of a newborn screening sample.[231] But the closely related section of the newborn screening regulations, entitled “Record Retention,” is silent regarding unrelated use of newborn screening records or the data they contain.[232] This section instead requires records, including the results of newborn screening, to “be kept for at least two years.”[233]
As is evident from these examples, states that have policies in place for samples but not for related data tend to be those that strictly limit secondary use of newborn screening samples. In one sense this disparity in regulatory attention may be logical. Current newborn screening methods do not produce very much genetic sequence data.[234] Conducting genetic research or identification for law enforcement purposes therefore would require access to the newborn screening sample, rather than the related data. Laboratory personnel and courts responding to law enforcement requests for access may therefore wish to construe this silence narrowly, even as screening tests and related efforts using genetic sequencing increase in number.[235]
b. Policies for Data, but Not Samples.—
More often, state policies have a rule in place for data but not necessarily for the samples themselves. For instance, as described supra in section II(B)(1), Connecticut, Hawaii, and Maryland each provide robust protection for newborn screening data.[236] But the statutory or regulatory frameworks in these states do not take the same care with respect to newborn screening samples themselves.
A policy governing data but not samples may also arise when the policy at issue is not newborn screening specific, but rather stems from more-general genetic privacy protections (and their exceptions). Nevada and New Jersey specifically exclude their newborn screening programs from protections otherwise available for “genetic information,” and permit such information to be obtained and disclosed for law enforcement purposes without consent.[237] In Minnesota, the state supreme court held that protections for “genetic information” apply not only to newborn screening data but also to the blood samples that give rise to that data.[238] Other states could adopt similar reasoning, yielding policies that apply to newborn screening resources equally, whether they are policies of restriction or of access.
c. Conflicting Policies for Samples and Data.—
Perhaps most oddly, some states have articulated policies for both samples and data, but the answer to the law-enforcement-access question diverges depending on which resource is at issue. Iowa is the most striking example. As set forth above, Iowa’s administrative code contains the clearest prohibition on law enforcement access to newborn screening samples, as well as arguable permission for law enforcement access to newborn screening data.[239] Both provisions appear in the regulations governing the newborn screening program itself.
Other states have also adopted diverging policies. Under New Mexico’s regulatory regime, “[b]loodspot cards shall not be disseminated after blood spot testing for any purpose unrelated to newborn screening, except to parents or guardians who may request them in writing during the retention period.”[240] Nonetheless, records and data held by the health department are “subject to subpoena for use in any pending cause in any administrative proceeding or in any of the courts of the state, unless otherwise provided by law.”[241] Similarly, in Delaware, “[d]ried blood-spots . . . will only be used for activities to improve the screening program and/or develop new screening tests.”[242] By contrast, genetic information may be disclosed where “necessary for the purposes of a criminal or death investigation or a criminal or juvenile proceeding,” when “authorized by order of a court of competent jurisdiction,” or “for the purpose of identifying bodies.”[243]
In both New Mexico and Delaware, the more stringent restriction appears in provisions specific to the newborn screening program directly, while the latter authorization appears in more general statutory sections. Accordingly, one could conclude that the newborn-screening-specific approach should control over the more general (and permissive) one. But that is not necessarily correct. After all, in both states, the two legal sources at issue are not inherently inconsistent, as one applies to samples and the other to data. The result is that in Delaware and New Mexico, newborn screening samples may be subject to different, and greater, privacy protections than the data drawn from them.
5. Standards for Law Enforcement Access.—
Even among states that contemplate law enforcement access to newborn screening samples, data, or genetic information more broadly, there is considerable divergence in the standards law enforcement must meet to obtain that access.
A few states appear to permit law enforcement access to newborn screening resources even in the absence of a court order. This is most likely to occur where law enforcement use of genetic information writ large is expressly contemplated, as in genetic privacy statutes that exclude from their protections any disclosures that are “necessary for the purposes of a criminal or death investigation or a criminal or juvenile proceeding.”[244]
Other states enshrine law enforcement access of indeterminate breadth or standard when they exclude from protection those disclosures “provided” or “required by law.”[245] These boilerplate exceptions may indicate that these states have incorporated law enforcement access through inadvertence, rather than through thoughtful consideration. Moreover, murkiness surrounding their scope may weigh against a capacious construction.
A handful of states are more demanding, permitting disclosures that might include those to law enforcement only upon a court order.[246] In some instances, these disclosure provisions are codified as part of the newborn screening program itself. For instance, in Massachusetts, the newborn screening regulations state that the program “shall not disclose newborn screening results or any information or patient identifiers . . . except to: . . . anyone authorized to receive such information pursuant to a court order.”[247] In other states, this permission arises as part of more general genetic privacy statutes, as in New York.[248]
Meanwhile, Texas arguably restricts law enforcement to warrants in accessing both newborn screening samples and related data.[249] Following the Beleno litigation and news reporting in Texas surrounding the nonconsensual retention and use of newborn screening resources, the Texas legislature amended the statutory framework governing the newborn screening program several times. The relevant statutory provisions now permit disclosures “as authorized by court order.”[250] But other sections of this statutory provision make clear that not just any court order will do. Rather, “[r]eports, records, and information obtained or developed” by the newborn screening program “are not subject to subpoena.”[251] Moreover, although newborn screening samples and related data may, in some circumstances, be disclosed for research purposes, disclosure is not permitted “for purposes related to forensic science.”[252] In light of these restrictions, a judicial warrant could satisfy the relevant statutory requirements for disclosure, while a less searching court order probably would not.
* * *
The landscape of policies that might affect law enforcement access to newborn screening samples and related data is highly fragmented, at times inconsistent, and often seemingly the product of inattention to a looming issue. Few states have focused on the question of law enforcement access to newborn screening resources. Most of the least restrictive law enforcement provisions appear not in newborn screening statutes or regulations at all, but rather in more general sources like genetic privacy statutes. Yet, in nearly one-third of states, law enforcement may be able to access newborn screening samples, related data, or both. It is not at all clear that this is the approach that states would—or should—choose were the question squarely presented.
III. Regulating Law Enforcement Access to Preserve Public Health
In view of the myriad and divergent policy approaches that states have adopted, perhaps no single policy approach can—or should—command adherence. Diversity in state policies is often identified as a feature, not a bug, of our federal system.[253]
But the stakes of failing to regulate law enforcement access to newborn screening samples and related data are substantial. As lawsuits like Bearder and Beleno make clear, some parents care deeply about whether and how their infants’ genetic data is retained and used by the state. Tellingly, in the wake of the Texas litigation, the newborn screening program again made headlines when reporters uncovered that Texas had given hundreds of newborn screening samples to the federal Armed Forces DNA Identification Laboratory to help create a national mitochondrial DNA database.[254]
In other words, one of these approaches is superior to the others. At a minimum, states must strive to clearly articulate a policy governing law enforcement access to newborn screening materials. As important, states should expressly reject law enforcement access in this context.
State legislatures and departments of health, as the prime policy makers involved, are best positioned to take the lead in advancing policy reform. Minnesota and Texas amended their newborn screening statutes to be more protective of patient privacy and parental consent in response to litigation (and threats of further litigation).[255] Other states could do so proactively. If states decline to act, moreover, the federal government should exercise its spending power, as it has in the past, to spur improvements in policy.[256]
Iowa has already demonstrated that clear policy excluding law enforcement use is practicable. Its regulation declares unequivocally, “[a] residual newborn screening specimen shall not be released to any person or entity for . . . law enforcement purposes or to establish a database for forensic identification.”[257] This statement is unambiguous, and it provides a model to which other states should look.[258] Connecticut’s statutory scheme, which precludes the disclosure or use of newborn screening data in court proceedings, provides an alternative approach to excluding use of newborn screening resources more broadly.[259]
This Part articulates three reasons for preferring law enforcement exclusion over law enforcement access. Subpart III(A) argues that such a policy is likely essential for maintaining public trust in newborn screening programs, particularly as these programs evolve to consider greater swaths of genetic data. Subpart III(B) establishes that prohibiting law enforcement access is most consonant with respect for persons, a core ethical and policy commitment undergirding public health programs like newborn screening. Finally, subpart III(C) suggests that a policy of law enforcement access to generate leads likely runs afoul of the Fourth Amendment’s prohibition on unreasonable searches and seizures. Law enforcement access, if permitted at all, must be predicated on a judicially authorized warrant that meets constitutional muster.
Until states act, these arguments should inform how laboratory personnel or courts respond to law enforcement requests for access. At a minimum, in the face of silence or ambiguity, these actors should avoid the grave constitutional concerns that warrantless law enforcement access to newborn screening resources would entail.[260]
A. Preserving Public Trust
Public trust is essential to the success of public health initiatives.[261] Indeed, as others have recognized, “public trust in public health officials, their messages, and the science upon which their messaging is based, contributes to the success of public health interventions.”[262] This trust is so crucial to public health efforts because protecting and improving the public health often requires community-wide participation. This is most obviously true in cases like vaccination against communicable diseases, where higher rates of community involvement can yield benefits even for those who are unable to be vaccinated.[263] But it is also true in other public health contexts, including physical fitness campaigns, smoking cessation efforts, and the like.[264]
Where trust ebbs, public health efforts can face decline or outright failure—and the health of individuals and of the community can suffer as a result. This has been the experience, for instance, with efforts to achieve and maintain herd immunity through vaccination against measles, mumps, and rubella (MMR).[265] The MMR vaccine is well tested, safe, effective, and has been in use for decades.[266] But in 1998, an article in the Lancet, a highly regarded medical journal, purported to link the vaccine to a higher incidence of autism.[267] Although the article was later retracted in its entirety,[268] it provoked “sensational headlines on the MMR vaccine, even in nontabloid newspapers.”[269] That furor fueled a growing distrust of the MMR vaccine, vaccine manufacturers, and medical professionals who advise patients to have themselves and their children immunized.[270] Distrust, in turn, led to a long-term decrease in MMR vaccination rates among some populations in the United States.[271] In the United Kingdom, MMR vaccination rates took fourteen years to rebound to pre-1998 levels.[272] Measles, which was previously eradicated in the United States in 2000, has experienced a resurgence.[273] Distrust, inflamed by sensationalized media and scientific fraud, generated concrete harms for both infected individuals and the broader community.
Distrust that undermines public health springs not only from scientific and media-driven mischief but also from concerns about overweening government power. Consider just one way that trust deficits have beset public health efforts during the COVID-19 pandemic. At the outset of the pandemic, many public health officials and technologists hailed digital contact tracing as an essential component for bringing the pandemic to heel.[274] Contact tracing, of course, is a well-established public health tool. Ordinarily, a trained contact tracer interviews an infected person to identify all other individuals with whom the infected person may have been in contact.[275] The contact tracer can then inform and monitor those contacts for symptoms of infection themselves.[276] Digital contact tracing efforts sought to replace the human contact tracer with a smartphone app that could alert individuals if they had been in close proximity to someone who subsequently tested positive for the virus that causes COVID-19.[277]
Yet these efforts met with dismal rates of adoption among the American public.[278] In part, this may have been due to reasonable doubts about the accuracy or efficacy of conducting contact tracing through smartphone proximity sensing or location tracking.[279] Distrust in government, however, also played a key role in driving low rates of adoption of digital contact tracing tools. In a recent study tracking the use of a COVID-19 contact tracing app, the factor yielding the greatest divergence in rate of uptake was whether an individual expressed “trust in government.”[280] In the United States, concerns about digital contact tracing prominently included whether law enforcement might gain access to sensitive location or proximity data for investigative use.[281] These concerns were not ill-founded. Some governments suggested using cell phone location data or apps to monitor compliance with social distancing requirements,[282] while law enforcement elsewhere were involved in enforcing such requirements.[283] On the whole, discussion of digital contact tracing evoked concerns about law enforcement surveillance running amok, more than they conjured the language of public health itself.[284] Digital contact tracing, even when designed with privacy in mind, was unable to escape its law enforcement connotations and the distrust those connotations generated.[285] Among the lessons that public health is sure to glean from the COVID-19 pandemic is how mistrust of government, particularly as a data steward, can disrupt public health efforts.
Against this backdrop, there can be little doubt that permitting broad, widescale law enforcement access to public health resources like newborn screening samples and related data would undermine the public’s trust. Health information is deeply sensitive and highly revealing—likely even more so than location information.[286] That description applies to newborn screening samples as well as their related data, particularly as those data entail more genetic sequence data or data about an ever-larger number of screened conditions. If public health could not succeed even in the midst of a pandemic because law enforcement power was the public’s frame for understanding, then newborn screening may be similarly imperiled if the public comes to see this existing public health program through a law enforcement lens. Permitting law enforcement to access newborn screening resources would rightly facilitate such a framing.
Similarly, the Supreme Court itself has recognized the link between maintaining trust, promoting health and healthcare, and excluding law enforcement. In Ferguson v. City of Charleston,[287] the Court held that it violated the Fourth Amendment to programmatically screen the urine of pregnant patients for drugs, and then make those test results available to law enforcement in order to coerce women who tested positive to enter drug treatment.[288] As the Ferguson Court explained, “[t]he reasonable expectation of privacy enjoyed by the typical patient undergoing diagnostic tests in a hospital is that the results of those tests will not be shared with nonmedical personnel without her consent.”[289] The Court went further, however, explicitly linking the involvement of law enforcement in a program with an underlying public health purpose to both distrust and the negative consequences of that distrust: “an intrusion on that expectation [of privacy] may have adverse consequences because it may deter patients from receiving needed medical care.”[290]
Other government entities have also acknowledged the importance of shielding important public health or safety efforts from ordinary law enforcement use in order to facilitate broad participation and public trust. Under federal law, federally funded researchers generating or using identifiable, sensitive data are subject to a Certificate of Confidentiality.[291] That Certificate appears to provide strong protection against disclosures for law enforcement purposes. Indeed, while generally researchers must disclose information as “required by Federal, State, or local laws,” that obligation does not apply to “any such information, document, or biospecimen that contains identifiable, sensitive information.”[292] Rather, researchers subject to a Certificate of Confidentiality are instructed not to disclose such data in “any Federal, State, or local civil, criminal, administrative, legislative, or other proceeding.”[293] More broadly, these data are “immune from the legal process.”[294]
Even CODIS itself—the FBI’s official DNA database for law enforcement purposes—has components that are walled off from ordinary crime-detection work. In addition to its databases of crime scene samples and known past offenders and arrestees, CODIS also contains databases pertinent to identifying missing persons.[295] These include databases of DNA profiles “recovered from unidentified human remains” and those “voluntarily contributed from relatives of missing persons.”[296] The last of these are known as “Family Reference Samples.”[297] As relevant here, according to the FBI, “[t]he DNA profiles obtained from the Family Reference Samples will only be searched against the DNA profiles from unidentified persons stored” in the national database.[298] Family Reference Samples, in other words, cannot be utilized for ordinary crime-detection purposes, for instance, by comparing DNA profiles from those samples to the DNA profiles believed to be from the perpetrators of unsolved crimes. Absent this limitation, perhaps some family members would be unwilling to cooperate as fully with law enforcement efforts to find or identify their loved ones. In order to encourage trust and participation from family members of missing persons, the FBI has tied its own hands.[299]
The blood samples used in newborn screening, like the urine screened in Ferguson, are obtained in a hospital as part of ordinary care. Their primary purpose is for diagnostic testing to determine whether a particular newborn is afflicted with a serious genetic condition that may successfully be halted with early intervention. The CDC has hailed newborn screening programs as “one of the nation’s most successful public health programs” precisely because of its comprehensive reach, including nearly all newborns each year.[300] Law enforcement entanglement with the newborn screening program, as would occur if law enforcement were able to access newborn screening resources for ordinary crime-detection purposes, would likely undermine trust in newborn screening all together, and perhaps drive some parents to decline or evade screening entirely. By contrast, restrictions on law enforcement access to newborn screening resources—like those applicable to “identifiable, sensitive information” in federally funded research or to Family Reference Samples in CODIS—would preserve and enhance public trust in these important public health programs.
B. Affirming Respect for Persons
Respect for persons is a core ethical commitment in American bioethics, medical ethics, and public health ethics.[301] This principle “incorporates at least two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection.”[302] The concept of respect for persons is one of four key principles that have guided much of American bioethics, including in the field of public health.[303]
Respect for persons most frequently finds expression in requirements for informed consent. As public health scholars have recognized, in giving concrete meaning to abstract moral considerations, “[a] common example is specifying respect for autonomy by rules of voluntary, informed consent.”[304] The same has emerged in clinical and research ethics.[305]
Informed consent is not the whole of respect for persons, however. Particularly in public health, “it would be a mistake to suppose that respect for autonomy requires consent in all contexts of public health or to assume that consent alone sufficiently specifies the duty to respect autonomy in public health settings.”[306] Indeed, in the context of newborn screening programs, states have often eschewed informed consent. Rather, states have asserted that their general public health powers or parens patriae authority authorize nonconsensual newborn screening, or at least permit consent to be presumed.[307]
Moreover, current federal research regulations do not require informed consent for most research uses of newborn screening samples or related data. Under the federal Common Rule, researchers need not seek separate consent for the research use of de-identified biospecimens that were initially collected for another purpose.[308] This classification reflects the Department of Health and Human Services’ judgment that, when disassociated from identifying information, the use of human biospecimens or genetic data in research is of sufficiently minimal risk (and sufficiently substantial utility) that it need not be considered human subjects research at all.[309] For this reason, the Common Rule requires periodic reconsideration of the meaning of “identifiable biospecimen” and “identifiable private information.”[310] As technology makes genetic data more and more easily re-identifiable (if it has not already done so), the scope of the Common Rule’s coverage should, in theory, expand.
But it is not clear that the Common Rule’s approach to subsequent use of newborn screening samples and related data is the correct one where research is at issue—much less law enforcement use. The Common Rule itself is ambivalent about the choice it has made in categorizing biospecimens and genetic data, as evidenced by its command for periodic reconsideration of the issue.[311] Lawsuits in Minnesota, Texas, and elsewhere over the nonconsensual retention and research use of newborn screening resources likewise indicate that there are limits to the accommodations that respect for persons will make in the absence of informed consent. In both Minnesota and Texas, parents demanded that the state destroy their children’s newborn screening samples so that they could not be used for research without consent.[312] In both cases, the plaintiffs were vindicated, whether by judicial decision or through settlement.[313] Thus, where pressed, states and courts have acknowledged that respect for persons may be inconsistent with the nonconsensual research use of newborn screening resources, even if consent is not required for the public health newborn screening itself.
Moreover, the justifications permitting nonconsensual newborn screening—or even subsequent nonconsensual research use of newborn screening resources—are simply inapplicable to law enforcement use of newborn screening resources. For one thing, it is nonsensical to imagine that the state can, in the exercise of its parens patriae power, consent to law enforcement searches for ordinary crime-detection purposes. Such an approach would conflict directly with existing legal doctrine governing consent to search, as well as that applicable to “special needs” searches, which permit suspicionless or programmatic searches only for purposes other than traditional law enforcement.[314]
For another, the de-identification and minimal risk analyses undergirding nonconsensual use of biospecimens for research do not carry over to law enforcement use. In order for law enforcement to make use of genetic data, it must be able to link that data to an identifiable person. Law enforcement’s use of newborn screening samples or related data is, accordingly, predicated on law enforcement’s ability to access identifiable, rather than de-identified, data. Nor can law enforcement’s use of newborn screening resources for crime-detection purposes reasonably be described as minimally risky. Individuals whom law enforcement come to suspect have committed crimes after comparing crime scene DNA to newborn screening resources may be deprived of their liberty. That is no small risk. Law enforcement use of newborn screening samples or related data thus derogates the principle of respect for persons and is inconsistent with existing justifications for permitting nonconsensual collection, retention, or other use of these resources.
More broadly, in place of informed consent, respect for persons in newborn screening programs may find expression elsewhere. Public health as a paradigm is characterized not only by consent-based cooperation, but also by “limiting both the information that government authorities collect and their use of that information.”[315] States have frequently adopted the language of minimization and privacy, asserting their respect for persons through assurances that newborn screening samples and related data will be “confidential.”[316] In so doing, states imply that they will guard these resources, confining their use to the purposes that justified their collection in the first instance. Routine law enforcement access to newborn screening resources for investigative purposes, by contrast, would run counter to such promises of confidentiality.
Permitting law enforcement to access newborn screening samples and related data for crime-detection purposes, in other words, is a serious affront to the core ethical principle of respect for persons. Such access would undermine the public health justifications of collecting, retaining, and even repurposing newborn screening resources for research use.[317] Even more than the parents in Bearder and Beleno, individuals whose children’s genetic data—or their own—is made available for law enforcement purposes without their consent would rightly conclude that the state has disregarded their autonomy. Respect for persons would be better aligned with closely controlling the subsequent uses to which newborn screening resources are put, and by explicitly excluding uses that could impose significant risk on individuals’ privacy or liberty interests.
C. Observing Constitutional Boundaries
In addition to concerns about undermining public trust or diminishing respect for persons—and thereby discouraging broad public support and participation in newborn screening—permitting law enforcement to access newborn screening resources without a warrant for crime-detection purposes is likely to run afoul of the Fourth Amendment to the U.S. Constitution. The Fourth Amendment guarantees the “right of the people to be secure in their persons, houses, papers, and effects against unreasonable searches and seizures.”[318] Ordinarily, the government must obtain a warrant, supported by probable cause, before conducting a search intended to discover evidence of criminal conduct.[319] As described below, warrantless law enforcement use of newborn screening resources violates this foundational precept, and such use is not immunized by either the “special needs” doctrine nor the “third party” doctrine.
As an initial matter, there can be no dispute that newborn sampling itself is a search subject to the Fourth Amendment. The Supreme Court has repeatedly held that blood tests constitute Fourth Amendment searches.[320] The Supreme Court has similarly explained that analysis of bodily fluids, including blood and urine, are searches.[321] Moreover, even if the initial blood collection is performed by a private, nongovernmental hospital, this will not immunize such conduct from Fourth Amendment scrutiny. After all, newborn screening is performed pursuant to government direction and to facilitate the state’s own analysis of the resulting blood sample.[322] In every relevant sense, hospital personnel act as government agents for purposes of obtaining newborn screening samples.[323] As described below, newborn screening itself is generally permissible consistent with the Fourth Amendment under the special needs doctrine. Law enforcement access to newborn screening resources, however, is not—and such access might well alter the constitutional analysis applicable to newborn screening in the first instance.
1. The “Special Needs” Doctrine Does Not Apply.—
Despite the general rule requiring a warrant prior to government search, the Supreme Court has identified several exceptions to the warrant requirement. Among these are the special needs cases. Under the special needs doctrine, the government may conduct even suspicionless, programmatic searches.[324] But such searches must achieve a reasonable balance when weighing “the intrusion on the individual’s interest in privacy against the ‘special needs’ that supported the program.”[325] In particular, these special needs must be justified by purposes “divorced from the State’s general interest in law enforcement.”[326] In cases in which the Supreme Court has approved searches under the special needs doctrine, “there were protections against the dissemination of [test] results to third parties,” including law enforcement.[327]
Indeed, the Court’s decision in Ferguson v. City of Charleston is persuasively on point. Ferguson rejected application of the special needs doctrine, and so helps to define the outer limits of that doctrine.[328] As explained above, Ferguson considered the constitutionality of a program to perform routine drug urinalysis on pregnant women seeking prenatal medical care—and then to turn positive drug test results over to law enforcement.[329] The Court did not question the authority and propriety of routinely screening pregnant patients for drug use, or of using the results of such tests to counsel women about drug treatment.[330] Rather, the Fourth Amendment violation occurred when hospital personnel shared those test results with law enforcement specifically.[331] Ferguson explained that, where law enforcement was party to the results of suspicionless searches and empowered to threaten criminal sanction, there could be no claim that such searches were “divorced from the State’s general interest in law enforcement.”[332] Rather, “[t]he Fourth Amendment’s general prohibition against nonconsensual, warrantless, and suspicionless searches necessarily applies to such a policy.”[333]
So too here. Existing newborn screening programs depend on the special needs doctrine. Newborn screening is suspicionless, programmatic, and population wide.[334] It is justified by substantial public health goals and achievements.[335] These goals are plainly divorced from that of ordinary law enforcement. Thus, newborn screening programs have traditionally fallen well within the bounds of the special needs doctrine.
But if law enforcement comes to make habitual or routine use of newborn screening resources for crime-detection purposes, that would disrupt the application of the special needs doctrine in this context. The collection, analysis, and retention of newborn screening samples and related data would no longer be “divorced from the State’s general interest in law enforcement.”[336] Law enforcement access to and use of newborn screening resources, in other words, might well taint an otherwise unobjectionable program, as occurred in Ferguson.
Nor is it sufficient to end-run the Fourth Amendment on the basis that law enforcement accessing newborn screening resources would be exploiting samples already collected or data already generated. In some cases, courts interpreting Ferguson have relied on such logic to hold that law enforcement access to medical data does not require a warrant because that data is generated by someone other than the government.[337] But there can be no dispute that the samples collected and data generated through newborn screening are searches attributable to the government. As explained above, hospital personnel collect newborn screening samples because they are required to do so by state law, and they typically transmit those samples to the state itself for analysis.[338] Newborn screening is also largely conducted without consent.[339] Whatever else might be said about the Fourth Amendment status of medical data generated within the scope of a doctor–patient relationship, the Fourth Amendment must apply where the state compels the one search and performs the other.[340] Even the dissenters in Ferguson acknowledged that, where the government is responsible for compelling a biospecimen, “the subsequent testing and reporting of the results to the police are obviously part of (or infected by) the same search.”[341] Moreover, in the context of the special needs doctrine, the Court has already suggested that such secondary use could “give rise to an inference of pretext, or otherwise impugn” an otherwise-constitutional special needs program.[342] Such a concern would rightly be raised in the context of newborn screening resources made available for law enforcement purposes.[343]
Finally, Maryland v. King[344] is not to the contrary. In King, the Supreme Court held that it is constitutionally permissible to compel and analyze a DNA sample for inclusion in CODIS from an individual arrested for, but not yet convicted of, a crime.[345] The Court based its conclusion on the State’s need to ascertain the “identity” of a person in custody.[346] Although the Court couched its opinion in language reminiscent of the special needs doctrine, it did not rely on that doctrine to uphold the challenged practice of DNA sampling of arrestees.[347] The Court instead relied on, among other things, the diminished privacy expectations of those who have been arrested.[348] As the Court recognized, similar searches of “the average citizen” would require “unique needs.”[349] Newborn screening itself, when put to its traditional public health goals, might present these “unique needs.” But newborn screening repurposed for law enforcement surveillance would not.
In sum, law enforcement access to or use of newborn screening resources cannot be justified under the well-established boundaries circumscribing the special needs doctrine. Such use would plainly be in service of “the State’s general interest in law enforcement.”[350] Moreover, increasing entanglement between newborn screening programs and law enforcement could well endanger the lawfulness of newborn screening programs in the first instance.
2. The “Third Party” Doctrine Does Not Apply.—
The Justices who dissented in Ferguson objected that the relevant conduct at issue in that case was not even a “search” within the meaning of the Fourth Amendment.[351] That determination drew on the logic of the so-called third party doctrine, which embodies a substantive limitation on the reach of the Fourth Amendment.[352] By its own terms, the Fourth Amendment regulates the government’s ability to conduct “searches” and “seizures.”[353] If the conduct at issue does not constitute a “search” or “seizure,” Fourth Amendment scrutiny simply does not apply. Since the Supreme Court’s decision in Katz v. United States,[354] to determine whether a “search” has occurred, courts principally inquire whether the place, thing, or information that the government seeks to examine is one in which an individual has an “expectation of privacy . . . that society is prepared to recognize as reasonable.”[355] Where there is such an expectation, government “intrusion into that private sphere generally qualifies as a search and requires a warrant supported by probable cause.”[356]
The third party doctrine emerged from two Supreme Court decisions in the 1970s that elaborated on how this “expectations of privacy” test should apply when an individual shares information with a third party from whom law enforcement subsequently obtains that information.[357] In Miller, the Court held that an individual could have no legitimate expectation of privacy in his bank records, and so there was no government “search” requiring Fourth Amendment scrutiny.[358] Similarly, in Smith, the Court concluded that the use of a pen register at the telephone company office to record telephone numbers dialed did not constitute a “search” subject to the Fourth Amendment either.[359] In both cases, the Court reasoned that the data at issue—bank records and telephone numbers—was not really private or confidential at all.[360] As important, the Court explained, the defendants had “voluntarily conveyed” the information at issue to a third party, and in so doing, “assumed the risk” that those records “would be divulged to police.”[361] In the decades that followed, lower courts often interpreted Miller and Smith as establishing a near-categorical rule: “if you share information, you do not have an expectation of privacy in it.”[362]
Even under this broad understanding, the third party doctrine would not immunize law enforcement access to newborn screening resources from Fourth Amendment scrutiny. That is so because, as emphasized above, newborn screening nearly always occurs without consent, either from the patient tested or her parents.[363] The third party doctrine, by contrast, is built on at least a kernel of voluntariness to share information with another. But even that kernel is absent from a program in which parents must take affirmative steps to avoid newborn screening, if such refusal is even permitted.
In recent years, moreover, the Supreme Court has also taken steps to curtail the broadest interpretations of the third party doctrine. Most significantly, in Carpenter v. United States,[364] the Court held that government access to a week’s worth of an individual’s historical cell phone location data—data that is compiled and held by cell phone companies—amounts to a search subject to the Fourth Amendment and typically requires a warrant.[365] In other words, cell phone location data is data in which an individual may maintain a reasonable expectation of privacy against government access and use.[366] Carpenter emphasized that the expectations of privacy test must reflect the Fourth Amendment’s goals “to secure the privacies of life against arbitrary power” and “to place obstacles in the way of a too permeating police surveillance.”[367]
Carpenter did not reject Miller and Smith; instead, it declined to “extend” them to cover historical cell phone location data.[368] In so doing, however, Carpenter did reject the categorical approach of the third party doctrine. Instead, the Court explained that cell phone location data differs from the bank records or telephone numbers at issue in the earlier cases along both axes undergirding the third party doctrine—the sensitivity (or lack thereof) of the information at issue and the voluntariness of its being shared with a third party.[369] Historical cell phone location data provides a pervasive, “time-stamped,” and “intimate window into a person’s life, revealing not only his particular movements, but through them his familial, political, professional, religious, and sexual associations.”[370] Moreover, that data is not genuinely “voluntarily shared,” in view of the ubiquity of cell phones in modern daily life and those phones’ transmission of location information as an automatic, largely invisible facet of their operation.[371]
Focusing on these characteristics, the Court identified three key factors informing its conclusion that individuals retain an expectation of privacy in their location information despite its third-party collection and storage: first, “the deeply revealing nature” of the information sought; second, the “depth, breadth, and comprehensive reach” of collections of such data; and third, “the inescapable and automatic nature of its collection.”[372] In addition, the Court emphasized that a more limited third party doctrine is particularly important where the government can make use of otherwise sensitive data in third-party hands in “remarkably easy, cheap, and efficient [ways] compared to traditional investigative tools,” such as “[w]ith just the click of a button.”[373]
Following Carpenter, there can be little doubt that law enforcement use of newborn screening resources would contravene a reasonable expectation of privacy. As I have explained elsewhere, genetic data is, by its nature, “deeply revealing” and presumptively private.[374] Many states acknowledge as much when they declare that newborn screening samples or related data will be “confidential.”[375]
The Supreme Court has similarly recognized that an individual may have a reasonable expectation of privacy in her genetic data. In Maryland v. King, although the Supreme Court held that no Fourth Amendment violation occurred, the Court considered the analysis of a compelled genetic sample to be a separate Fourth Amendment event from the acquisition of the sample itself.[376] This separate consideration of genetic analysis indicates that genetic data carries with it an enduring privacy interest of constitutional magnitude.[377] That the Court treated genetic analysis as a separate search is all the more telling because the genetic data extracted in King was limited to the data points used to compile a CODIS profile.[378] The genetic markers used in CODIS profiles are “designed to be maximally informative about individual identity, but minimally informative about anything else.”[379] The Court in King specifically reserved the question of whether the Fourth Amendment proscribes law enforcement access to genetic analysis related to, “for instance, an arrestee’s predisposition for a particular disease or other hereditary factors not relevant to identity,” explaining that such data “would present additional privacy concerns.”[380]
Yet these richer data are precisely the kind of data created through newborn screening. Newborn screening programs are designed explicitly to identify children who are affected with particular genetic diseases.[381] Were genome-wide sequencing in newborn screening to become the norm, it would exponentially increase the range and sensitivity of the data such screening generates. Law enforcement use of newborn screening data would accordingly tread on privacy interests the King Court was unwilling to require even arrestees to forgo.
Moreover, even if law enforcement limited its use of newborn screening resources to compiling CODIS-related genetic data, that limitation would not insulate law enforcement use from renewed Fourth Amendment scrutiny. For one thing, the Court in Carpenter declined to hold that the Fourth Amendment was satisfied (or obviated) where law enforcement obtained only a limited set of the available location data.[382] Extracting a limited set of sensitive information from a larger whole of such data is likely insufficient to address the Fourth Amendment. For another, King approved warrantless DNA sampling and analysis based on the generally diminished expectations of privacy that arrestees may maintain.[383] By contrast, the Court recognized that similar searches of “the average citizen” would require further scrutiny.[384] Newborn screening samples and related data, collected from nearly every newborn American citizen, thus cannot pass muster based on King, no matter how limited the genetic data involved.
Newborn screening resources are not merely “deeply revealing” on an individual level; they are also subject to deep, broad, and comprehensive collection.[385] Newborn screening data is deep in the sense that it reveals highly detailed and precise information about an individual, as it is used to diagnose serious genetic disorders.[386] More generally, genetic data can be both highly detailed and precise at an individual level. That is why DNA has been lauded as the gold standard for forensic identification.[387] Genetic data is also broad: “[A] single cell contains the whole of an individual’s genetic information.”[388] To be sure, an individual’s genetic data does not vary substantially over time, and so it is not “broad” in quite the same sense as location data, the collection of which paints a detailed portrait of an individual’s movements through time and space.[389] But genetic data, like location data, is broad in the scope and range of information it can disclose. For both newborn screening samples and genome-wide genetic data, that information can reveal not only current and potential health risks, but also an individual’s identity, genetic relatedness, and physical traits.[390] Finally, newborn screening resources are subject to comprehensive collection and often retention over years or even decades, as newborn screening programs reach the vast majority of children born in the United States each year.[391]
Turning to Carpenter’s third factor, “the inescapable and automatic nature of its collection,”[392] it is difficult to imagine a more paradigmatic example than newborn screening. Newborn screening programs attempt to reach every infant born in the United States—and they largely succeed.[393] They do so because newborn screening is a standard part of newborn care that is mandated by law for every infant born in a hospital, birthing center, or with a midwife.[394] In this sense, newborn screening is more inescapable than the collection of cell phone location data at issue in Carpenter.[395] If one’s need to have and use a cell phone as a part of modern life renders that phone (and its generation of location data) inescapable, [396] then surely the need for assistance to birth a child is similarly inescapable. Newborn screening is also largely “automatic,” as standard newborn care to which new parents are not asked affirmatively to consent and to which they must instead affirmatively object.[397]
Finally, consider the efficiency gains that law enforcement use of newborn screening resources may make possible. At present, newborn screening data may be of little interest to law enforcement, as these data contain little genetic sequence information.[398] But increasing use of genetic sequencing in newborn screening, a more dramatic expansion into genome-wide sequencing for newborns, or widespread efforts akin to authentication of biospecimens would potentially put highly identifiable, individual level genetic data for virtually all Americans into databases within the government’s grasp. In the absence of Fourth Amendment or other warrant requirements, all Americans’ genetic data would be available “[w]ith just the click of a button.”[399] Even without genetic sequence data, the collection and retention of newborn screening samples over long periods of time could enable law enforcement to realize dramatic efficiency gains. If a mere request or subpoena might pry open the Health Department’s drawers, then law enforcement may more easily build troves of genetic data outside of CODIS for use in solving crimes.[400] That was not the intent of newborn screening programs, and it threatens to obviate the serious and substantial limitations state and federal governments have put in place regarding CODIS itself. Moreover, it portends the sort of “too permeating police surveillance” that Carpenter guards against.[401]
In sum, law enforcement use of newborn screening resources demands vigorous Fourth Amendment scrutiny. Neither the special needs doctrine nor the third party doctrine can shield such use from the Fourth Amendment’s traditional requirement for a warrant. Any less potent means of access—whether by request, subpoena, or non-warrant court order—is inconsistent with that protection and should be unlawful.[402]
Conclusion
Newborn screening programs have been a public health success story. But growing law enforcement interest in using genetic data beyond CODIS to solve crimes may soon undermine these crucial public health programs. Nearly one-third of states arguably permit newborn screening samples or their related data to be disclosed to or used by law enforcement. More than a quarter of states have no discernible policy in place regarding law enforcement access whatsoever. Even among those states that have clearly excluded law enforcement from acceptable use of either newborn screening samples or data, a few nonetheless have policies permitting law enforcement access to the other. For some states across these groups, regulatory provisions do not require a warrant to compel disclosure. In many instances, policies regulating law enforcement access appear to emerge from inattention to this looming and growing issue.
Yet a warrant is the constitutional minimum that law enforcement should be required to secure before accessing newborn screening resources. Better yet, to preserve public trust in newborn screening programs and to conform with the foundational principle of respect for persons, law enforcement should clearly and unequivocally be barred from access. Every newborn American is counting on it.
- .See Newborn Screening 101, Baby’s First Test, https://www.babysfirsttest.org/newborn-screening/screening-101 [https://perma.cc/27GZ-CUSQ] (last updated Aug. 30, 2021) (“Newborn screening is a state public health service that reaches each of the nearly 4 million babies born in the United States each year.”). ↑
- .How Is Newborn Screening Done?, Nat’l Inst. Child Health & Hum. Dev., U.S. Dep’t Health & Hum. Servs. (Sept. 1, 2017), https://www.nichd.nih.gov/health/topics/newborn/conditioninfo/how-done#f1 [https://perma.cc/9QBK-Q7RU]. ↑
- .See Recommended Uniform Screening Panel, Health Res. & Servs. Admin. (Feb. 2020), https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html [https://perma.cc/DG83-KRDE] (identifying conditions that the Secretary of the Department of Health and Human Services recommends for newborn screening); Newborn Screening 101, supra note 1 (“Most states screen for at least 31 of the 35 conditions recommended by the Advisory Committee on Heritable Disorders in Newborns and Children.”). ↑
- .See Newborn Screening 101, supra note 1 (explaining that newborn screening permits diagnosis and treatment before symptom onset and that “[m]ost affected babies identified through newborn screening who receive treatment early grow up healthy with normal development,” but “once symptoms appear, they are often irreversible, leading to severe health and developmental problems or even death”). ↑
- .See How Many Newborns Are Screened in the United States?, Nat’l Inst. Child Health & Hum. Dev., U.S. Dep’t of Health & Hum. Servs. (Sept. 1, 2017), https://www.nichd.nih.gov/health/topics/newborn/conditioninfo/infants-screened#f [https://perma.cc/WC7W-Z636] (“The latest CDC data show that about 12,500 newborns each year are diagnosed with one of the core conditions detected through newborn screening.”). ↑
- .Newborn Screening Laboratory Bulletin, Ctrs. for Disease Control & Prevention (Feb. 21, 2014), https://www.cdc.gov/nbslabbulletin/bulletin.html [https://perma.cc/4PY2-4R8X] (“Newborn screening is one of the nation’s most successful public health programs.”). ↑
- .See Sonia M. Suter, Did You Give the Government Your Baby’s DNA? Rethinking Consent in Newborn Screening, 15 Minn. J.L. Sci. & Tech. 729, 754 (2014) (indicating that residual blood remains after newborn blood spots are analyzed). ↑
- .See Michelle H. Lewis, Aaron Goldenberg, Rebecca Anderson, Erin Rothwell & Jeffrey Botkin, State Laws Regarding the Retention and Use of Residual Newborn Screening Blood Samples, 127 Pediatrics 703, 705–06 (2011) (describing the potential uses for residual dried-blood samples); Suter, supra note 7, at 75455 (indicating that states retain newborn blood samples for analysis). ↑
- .Suter, supra note 7, at 754 (“Some states have provisions to retain samples for only one to four weeks, some for months, some for years, some for decades, and others indefinitely.”). ↑
- .See Julie Watts, California Stores DNA from Every Baby: Renewed DNA Privacy Concerns Following SFPD Rape-Kit Allegations, CBS Sacramento (Feb. 16, 2022, 9:22 AM), https://sacramento.cbslocal.com/2022/02/16/california-biobank-dna-privacy-concerns/ [https://perma.cc/L672-M4CE] (“[B]lood spots are being used by law enforcement. We found at least five search warrants and four court orders for identified blood spots before the Golden State Killer case . . . . Since then, investigators have confirmed newborn blood spots are being used to solve cold cases.”). ↑
- .See Natalie Ram, Genetic Privacy After Carpenter, 105 Va. L. Rev. 1357, 1359–61 (2019) [hereinafter Ram, Genetic Privacy] (describing the Golden State Killer investigation and the resulting increase in similar investigations that soon followed). ↑
- .Id. at 1359. ↑
- .See Parabon Tops 200 Solved Cases, Parabon NanoLabs (Jan. 19, 2022), https://parabon-nanolabs.com/news-events/2022/01/parabon-tops-200-solved-cases.html [https://perma.cc/5TBK-KRH2] (claiming that, in 200 cases, Parabon efforts have yielded “a lead [that] . . . resulted in a positive identification”); see also Natalie Ram, Investigative Genetic Genealogy and the Future of Genetic Privacy, SciTech Law., Summer 2020, at 18, 19 [hereinafter Ram, Investigative Genetic Genealogy] (“Parabon Nanolabs, the first private company to capitalize on law enforcement interest in investigative genetic genealogy, claims that it has already identified more than 100 suspects this way.”). ↑
- .See Julie Watts, CBS13 Investigates: CA Still Storing Newborn DNA Without Consent. Golden State Killer Case Raising New Concerns, CBS Sacramento (Dec. 7, 2020, 1:09 PM), https://sacramento.cbslocal.com/2020/12/07/newborn-dna-california-consent-gsk-killer/ [https://perma.cc/LK9H-BK4A] [hereinafter Watts, CBS13 Investigates] (“[B]lood spots are being used by law enforcement. We found at least five search warrants and four court orders for identified blood spots before the Golden State Killer case. Since then, . . . at least one cold case was recently solved with the help of newborn blood spots.”). ↑
- .Id. ↑
- .See Shawn E. McCandless & Erica J. Wright, Mandatory Newborn Screening in the United States: History, Current Status, and Existential Challenges, 112 Birth Defects Rsch. 350, 36061 (2020) (summarizing methods used by state labs to screen Recommended Uniform Screening Panel disorders). ↑
- .The Ethics of Sequencing Newborns: Reflections and Recommendations, Hastings Ctr., https://www.thehastingscenter.org/publications-resources/special-reports-2/ethics-sequencing-newborns-reflections-recommendations/ [https://perma.cc/Y7TF-KKAK]; see generally Symposium, The Ethics of Sequencing Newborns: Reflections and Recommendations, Hastings Ctr. Rep., July–Aug. 2018 (exploring ethical issues surrounding newborn sequencing); see also Jaime S. King & Monica E. Smith, Whole-Genome Screening of Newborns? The Constitutional Boundaries of State Newborn Screening Programs, Pediatrics, Jan. 2016, at S1, S12 (contemplating the use of whole-genome sequencing in newborn screening); infra notes 80–85 and accompanying text (discussing newborn genome sequencing efforts in more detail). ↑
- .See Suter, supra note 7, at 755 n.133 (observing that “[s]ome have called for universal DNA databanking for criminal forensic purposes,” and that newborn screening “blood spots would offer an easy way to achieve this goal”). ↑
- .See, e.g., Gretchen Smith, Debra Matthews, Samuel Sander-Effron, Deborah Requesens, Nahid Turan & Laura Schienfeldt, Microsatellite Markers in Biobanking: A New Multiplexed Assay, 19 Biopreservation & Biobanking 438, 438–39 (2021) (describing that current cell line authentication standards utilize some of the same genetic markers as law enforcement and introducing an alternative marker set that does not overlap with those used for law enforcement); Watts, supra note 10 (explaining that, although California “does not extract or sequence the DNA” as a part of current newborn screening, “a researcher or investigator may”). ↑
- .See Ram, Genetic Privacy, supra note 11, at 138081 (discussing reasonable expectations of privacy that users of consumer-genetics platforms may retain in their genetic data in light of platform privacy practices); Paige St. John, The Untold Story of How the Golden State Killer Was Found: A Covert Operation and Private DNA, L.A. Times (Dec. 8, 2020, 5:00 AM), https://www.latimes.com/california/story/2020-12-08/man-in-the-window [https://perma.cc/E6FS-AESN] (describing how investigators in the Golden State Killer case obfuscated the true extent of their use of consumer-genetics platforms and that, in fact, “[t]he actual investigation was broader and more invasive, conducted without a warrant, and appeared to violate the privacy policy of at least one DNA company”). Recent revelations about the San Francisco Police Department’s use of genetic data from sexual assault victims to identify them as suspects in unrelated crimes further underscore both law enforcement’s appetite to use genetic data from more sources to identify suspects and the disjunction between contributors’ expectations and law enforcement’s use. See Tami Abdollah, Rape Survivors, Child Victims, Consensual Sex Partners: San Francisco Police Have Used DNA from All of Them for 7 Years, USA Today (Feb. 25, 2022, 1:58 PM), https:// www.usatoday.com/story/news/nation/2022/02/23/san-francisco-police-rape-kit-dna-controversy/ 6854467001/ [https://perma.cc/SE46-2V9V] (documenting the “San Francisco police crime lab’s mixing of victim DNA samples in a broader local database”). ↑
- .See, e.g., Ellen Wright Clayton, Screening and Treatment of Newborns, 29 Hous. L. Rev. 85, 87 (1992) (“This Article argues that society should resist efforts to require that newborns be tested for an ever-increasing number of conditions.”); Katherine Drabiak-Syed, Legal Regulation of Banking Newborn Blood Spots for Research: How Bearder and Beleno Resolved the Question of Consent, 11 Hous. J. Health L. & Pol’y 1, 4 (2011) (discussing parental-consent requirements of states with respect to newborn screening); King & Smith, supra note 17, at S9 (examining the constitutional foundations of state-imposed newborn screening programs and the role of parental consent in light of advancements in whole-genome sequencing); Lewis, supra note 8, at 705 (surveying state laws governing retention and use of newborn screening samples and related data for research purposes); Suter, supra note 7, at 730 (discussing consent for screening, retention, and future use). ↑
- .In 2003, the U.S. General Accounting Office (GAO), now referred to as the Government Accountability Office, surveyed state statutes governing newborn screening programs, including those permitting disclosure “[i]n connection with law enforcement or legal proceedings.” U.S. Gen. Acct. Off., GAO-03-449, Newborn Screening: Characteristics of State Programs 24, 25 tbl.6 (2003) [hereinafter 2003 GAO Report], https://www.gao.gov/new.items/d03449.pdf [https://perma.cc/TN6U-UQ2C]. Given changes in screening technology and legal proceedings surrounding newborn screening programs, at a minimum, the GAO data is in need of updating and elaboration. See infra subpart I(A). ↑
- .See, e.g., Ellen Wright Clayton, Barbara J. Evans, James W. Hazel & Mark A. Rothstein, The Law of Genetic Privacy: Applications, Implications, and Limitations, 6 J.L. & Biosciences 1, 2022 (2019) (describing how genetic information generated for health-related purposes may be put to secondary use through permissive or compelled disclosure, including for “[c]riminal [j]ustice and [f]orensics”). ↑
- .See McCandless & Wright, supra note 16, at 350 (chronicling the development of newborn screening programs in the mid-1960s). ↑
- .How Is Newborn Screening Done?, supra note 2. ↑
- .See Suter, supra note 7, at 734 (explaining that Guthrie cards are used in newborn screening to analyze the newborn’s blood for different health conditions). Guthrie cards are named after Dr. Robert Guthrie. Dr. Guthrie developed the first newborn screening assay for phenylketonuria and the filter cards on which the blood for screening would be collected. Ellen Wright Clayton, Currents in Contemporary Ethics: State Run Newborn Screening in the Genomic Era, or How to Avoid Drowning When Drinking from a Fire Hose, 38 J.L. Med. & Ethics 697, 697 (2010) [hereinafter Clayton, Currents in Contemporary Ethics]. ↑
- .Suter, supra note 7, at 735. ↑
- .Nancy S. Green, Siobhan M. Dolan & Thomas H. Murray, Newborn Screening: Complexities in Universal Genetic Testing, 96 Am. J. Pub. Health 1955, 1955 (2006). ↑
- .Suter, supra note 7, at 735. ↑
- .Green, supra note 28, at 1955. ↑
- .Id. ↑
- .Clayton, Currents in Contemporary Ethics, supra note 26, at 697; McCandless & Wright, supra note 16, at 352. ↑
- .See McCandless & Wright, supra note 16, at 352 (“The implementation of screening, and the conditions screened, were determined state by state, with marked variability in the what and the how of testing . . . .”). ↑
- .2003 GAO Report, supra note 22, at 2. ↑
- .Suter, supra note 7, at 73637. ↑
- .See McCandless & Wright, supra note 16, at 358 (describing how, for the nearly thirty years following the introduction of newborn screening programs, separate tests were required for each condition). ↑
- .Id. at 352. ↑
- .Michael S. Watson, Marie Y. Mann, Michele A. Lloyd-Puryear, Piero Ronaldo, R. Rodney Howell, Am. Coll. of Med. Genetics Newborn Screening Expert Grp., Newborn Screening: Toward a Uniform Screening Panel and System—Executive Summary, 117 Pediatrics S296 (2006) [hereinafter ACMG Report]; see also Suter, supra note 7, at 737 (describing the ACMG recommendations). ↑
- .ACMG Report, supra note 38, at S302. These baseline criteria for effective early detection and treatment reflect similar priorities to earlier guidance from the World Health Organization and is known colloquially as the “Wilson and Jungner criteria.” McCandless & Wright, supra note 16, at 351 (internal quotation marks omitted). ↑
- .Newborn Screening Saves Lives Act, Pub. L. No. 110-204, 122 Stat. 705. ↑
- .See McCandless & Wright, supra note 16, at 353 (describing the Newborn Screening Saves Lives Act). ↑
- .Id. ↑
- .Id. ↑
- .Recommended Uniform Screening Panel, Health Res. & Servs. Admin. (Feb. 2020), https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html [https://perma.cc/SDZ6-EG6M]. ↑
- .McCandless & Wright, supra note 16, at 353. ↑
- .See Clayton, Currents in Contemporary Ethics, supra note 26, at 697 (describing the rapid adoption of early newborn screening programs, “which were almost always mandatory”); Suter, supra note 7, at 745–46 (discussing and critiquing the lack of affirmative informed parental consent for newborn screening). Suter identified two jurisdictions that required affirmative consent at the time of publication (D.C. and Wyoming) and observed that Maryland had recently moved from an opt-in to an opt-out approach. Id. at 747 & n.81. In the intervening years, D.C. has now also moved to an opt-out approach. See D.C. Code § 7-858.02(a) (2020) (“Each hospital, birthing facility, and nurse-midwife shall . . . [s]creen all newborns delivered or cared for at the hospital, home, or birthing facility for critical congenital heart disease, hearing impairment, and metabolic disorders, unless the newborn’s parent withholds consent for the screening procedure . . . .”) (emphasis added). ↑
- .McCandless & Wright, supra note 16, at 362. Even where parents are nominally empowered to opt out of newborn screening, lack of information and awareness that the screening will occur undermines this authority. See Suter, supra note 7, at 747 (“[P]arents are often woefully uninformed about [newborn screening].”). ↑
- .As Sonia Suter observes, this lack of informed consent is particularly unusual because newborn screening “is essentially a form of genetic screening.” Suter, supra note 7, at 748. “Mandatory genetic testing is extremely unusual,” Suter notes, “in large part because a strong consensus has existed for some time that genetic screening programs should not be compulsory and should involve informed consent.” Id.; see also Ruth Faden, Judith Chwalow, Neil A. Holtzman & Susan D. Horn, A Survey to Evaluate Parental Consent as Public Policy for Neonatal Screening, 72 Am. J. Pub. Health 1347, 1347 (1982) (“Despite this pervasive, routinely accepted practice [of newborn screening without parental consent], the overwhelming conviction in the literature on genetic policy is that compulsory genetic programs are inappropriate.”). ↑
- .See, e.g., Faden, supra note 48, at 1347 (observing that, in the context of newborn screening, “the exigencies of the situation are such that there is usually no opportunity for parents to voice objections”). ↑
- .See King & Smith, supra note 17, at S9 (“State-mandated [newborn screening] involves both health and children, so the police power and the parens patriae power work in combination to justify the state’s ability to require screening.”); Suter, supra note 7, at 75051 (identifying and critiquing both the police power and parens patriae bases for modern mandatory newborn screening programs). ↑
- .Lawrence B. Custer, The Origins of the Doctrine of Parens Patriae, 27 Emory L.J. 195, 195 (1978). ↑
- .See, e.g., Clayton, supra note 21, at 12829 (explaining the “problems with viewing the state as ‘parent’”); King & Smith, supra note 17, at S10 (“The inclusion of multiplex capability in the initial selection criteria set an undesirable precedent for future evaluation because it is not related to the child’s benefit or the state interests that support use of the police or parens patriae powers.”); Suter, supra note 7, at 75152 (discussing why “the parens patriae rationale is somewhat questionable”). ↑
- .The nomenclature for these blood samples varies. Often, they are described as “dried blood spots,” or “DBS.” Suter, supra note 7, at 731. They may also be described as “residual newborn screening blood samples” or “residual dried blood samples.” Lewis, supra note 8, at 703. To keep clear the relationship between newborn screening and the subsequent retention and use of the screening blood samples and the data developed from them, this Article largely refers to these resources as “newborn screening samples” and “newborn screening data.” ↑
- .See Lewis, supra note 8, at 705 (describing the various ways states regulate newborn blood spots). ↑
- .Id. ↑
- .Suter, supra note 7, at 754; see also, e.g., 16-4100-4107 Del. Admin. Code § 9.3 (2019) (“Dried blood-spots will be retained for a period of three years under appropriate conditions.”); Md. Code Regs. 10.10.13.15(D)(2) (2022) (“The Department’s public health laboratory shall retain and maintain for 25 years a newborn infant’s blood-spot specimen after the blood-spot specimen is received . . . .”); Minn. Stat. Ann. § 144.125 (West, 2021) (permitting the State Department of Health to “store blood samples and test results” without any end date unless a parent or legal guardian requests destruction); Tenn. Code Ann. § 68-5-406 (West, 2021) (requiring, in general, that a “newborn screening specimen taken for testing” be retained for “one (1) year,” but permitting longer retention in some circumstances); Michigan BioTrust for Health, Mich. Dep’t Health & Hum. Servs., https://www.michigan.gov/mdhhs/0,5885,7-339-73971_4911_4916_53246-209738—,00.html [https://perma.cc/6PC8-D9ZH] (“After newborn screening is performed at the state laboratory, any unused blood spots (including parts of blood spots) are stored for up to 100 years unless a parent or grown child (18 years or older) opts-out.”). ↑
- .Suter, supra note 7, at 756. ↑
- .Lewis, supra note 8, at 705. ↑
- .Id. at 706. ↑
- .See Suter, supra note 7, at 756–57 (“Very few states have specific regulations governing what kind of future uses the samples may be put to or requiring that parents be notified of or give consent for such uses.”). ↑
- .See Complaint at 2, Bearder v. Minnesota, No. 27-CV-09-5615, 2009 WL 4893192 (Minn. Dist. Ct. 2009) (arguing that “[d]efendants stored baby’s blood and genetic information without the consent of the baby’s parents, despite Minnesota law that requires consent”); Complaint at 36, Beleno v. Tex. Dep’t of State Health Servs., No. SA-09CA-0188-FB (W.D. Tex. 2009) (arguing that collecting newborn blood and holding it indefinitely without parental consent violated several federal and state laws). In 2018, a similar lawsuit was filed in Michigan. Kanuszewski v. Mich. Dep’t of Health & Hum. Servs., 927 F.3d 396 (6th Cir. 2019). At the time of writing, that litigation remains ongoing. ↑
- .Beleno v. Tex. Dep’t of State Health Servs., No. SA-09-CA-0188-FB (W.D. Tex. 2009). ↑
- .Suter, supra note 7, at 733 n.18, 75759 (describing the Beleno litigation). ↑
- .Bearder v. Minnesota, 806 N.W.2d 766 (Minn. 2011). ↑
- .Id. at 776. ↑
- .See Newborn Screening Program Information: Blood Spots and Test Results: Retention Practices, Minn. Dep’t of Health, https://www.health.state.mn.us/people/newbornscreening/program/retention.html [https://perma.cc/U8LN-UHWK] (“Newborn screening bloodspots collected before August 1, 2014 and their test results have been destroyed according to earlier statutory requirements.”). ↑
- .Minn. Stat. Ann. § 144.125(8)(10) (West 2021). ↑
- .Newborn Screening Saves Lives Reauthorization Act of 2014, 42 U.S.C. § 289. ↑
- .Id. ↑
- .2018 Requirements FAQs: Newborn Blood Spot, Off. for Hum. Rsch. Prots. (Jan. 16, 2019), https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/2018-requirements-faqs/index.html [https://perma.cc/96T6-HJ8M]. Section 12 of the Newborn Screening Saves Lives Reauthorization Act of 2014 specified that its classification of newborn screening samples as “human subjects” would persist only until updates and amendments to the Common Rule (already in progress) were promulgated. 42 U.S.C. § 289. ↑
- .Drabiak-Syed, supra note 21, at 4. ↑
- .See 2003 GAO Report, supra note 22, at 9 tbl.1 (identifying most commonly screened disorders). Like PKU, other rare inherited genetic disorders, including metabolic disorders (like galactosemia) and hemoglobinopathies (like sickle cell disease) are common in newborn screening programs. Id. But not all disorders subject to newborn screening are likely to be inherited from one’s parents. For instance, as early as 2003, all states screened for congenital hypothyroidism, which may be—but usually is not—inherited. See S.M. Park & V.K.K. Chatterjee, Genetics of Congenital Hypothyroidism, 42 J. Med. Genetics 379, 379 (2005) (describing genetic basis of congenital hypothyroidism and observing that “[m]ost cases of congenital hypothyroidism occur sporadically”). ↑
- .See Mary Ann Baily & Thomas H. Murray, Ethics, Evidence, and Cost in Newborn Screening, Hastings Ctr. Rep., MayJune 2008, at 23, 25 (“Tandem mass spectrometry measures the levels of various metabolites in the blood, and abnormalities in the levels suggest the presence of metabolic disorders.”); Suter, supra note 7, at 743 (describing “tandem mass spectrometry, which looks for a group of core conditions by identifying unusually high levels of metabolites related to these conditions”). ↑
- .McCandless & Wright, supra note 16, at 358. ↑
- .Id. ↑
- .Id. ↑
- .Id. ↑
- .Id. at 361 tbl.2. ↑
- .Id. at 359. ↑
- .See David R. Murdock, Frank X. Donovan, Settara C. Chandrasekharappa, Nicole Banks, Carolyn Bondy, Maximillian Muenke & Paul Kruszka, Whole-Exome Sequencing for Diagnosis of Turner Syndrome: Toward Next-Generation Sequencing and Newborn Screening, 102 J. Clinical Endocrinology & Metabolism 1529, 153536 (2017) (“[W]e feel our results demonstrate the ability of [whole-exome-sequencing]-based techniques to detect [Turner Syndrome], which strengthens the argument for the incorporation of next-generation sequencing into standard [newborn screening].”); Ingrid A. Holm, Pankaj B. Agrawal, Ozge Ceyhan-Birsoy, Kurt D. Christensen, Shawn Fayer, Leslie A. Frankel, Casie A. Genetti, Joel B. Krier, Rebecca C. LaMay, Harvey L. Levy, Amy L. McGuire, Richard B. Parad, Peter J. Park, Stacey Pereira, Heidi L. Rehm, Talia S. Schwartz, Susan E. Waisbren, Timothy W. Yu, The BabySeq Project Team, Robert C. Green & Alan H. Beggs, The BabySeq Project: Implementing Genomic Sequencing in Newborns, BMC Pediatrics, July 2018, at 1 (discussing the potential benefits of genomic sequencing). ↑
- .See generally King & Smith, supra note 17 (discussing constitutional implications of whole-genome sequencing); see also Holm, supra note 80, at 8 (describing BabySeq, a pilot project involving whole-exome sequencing of newborns). The “exome” refers to the coding part of DNA, while the “genome” includes both coding and noncoding portions. See Bahareh Rabbani, Mustafa Tekin & Nejat Mahdieh, The Promise of Whole-Exome Sequencing in Medical Genetics, 59 J. Hum. Genetics 5, 12 (2014) (discussing whole-genome sequencing and exome). Coding DNA, in turn, means the portion of DNA that “contains the information required to make proteins,” while “noncoding DNA does not encode for proteins and until recently was considered to have no biological function.” Natalie Ram, DNA by the Entirety, 115 Colum. L. Rev. 873, 880 (2015) [hereinafter Ram, DNA by the Entirety]. BabySeq may utilize whole-exome sequencing because it is currently less expensive than whole-genome sequencing, although it is not clear how long that differential in cost will persist. See Aziz Belkadi, Alexandre Bolze, Yuval Itan, Aurélie Cobat, Quentin B. Vincent, Alexander Antipenko, Lei Shank, Bertrand Boisson & Jean-Laurent Casanova, Whole-Genome Sequencing Is More Powerful than Whole-Exome Sequencing for Detecting Exome Variants, 112 Proc. Nat’l Acad. Scis. 5473, 5477 (2015) (noting the cost of whole-genome sequencing is expected to drop faster than the cost of whole-exome sequencing). For simplicity, this Article refers to whole-genome and whole-exome sequencing as “genome-wide sequencing.” ↑
- .Leslie G. Biesecker, Eric D. Green, Teri Manolio, Benjamin D. Solomon & David Curtis, Should All Babies Have Their Genome Sequenced at Birth?, 375 Brit. Med. J. 306, 306. In 2009, Dr. Francis Collins, then-Director of the NIH, similarly contended, “whether you like it or not, a complete sequencing of newborns is not far away.” Molly McElroy, Researchers and Policymakers Point to Successes and Challenges in Personalized Medicine, Am. Ass’n for Advancement of Sci. (Dec. 14, 2009), https://www.aaas.org/news/researchers-and-policymakers-point-successes-and-challenges-personalized-medicine [https://perma.cc/YF4H-8W97]. ↑
- .Biesecker, supra note 82. ↑
- .See NIH Program Explores the Use of Genomic Sequencing in Newborn Healthcare, Nat’l Insts. of Health (Sept. 4, 2013), https://www.nih.gov/news-events/news-releases/nih-program-explores-use-genomic-sequencing-newborn-healthcare [https://perma.cc/L4DQ-VJUH] (describing the program); Helen Thomson, Baby Genes to Be Mapped at Birth in Medical First, New Scientist (Apr. 8, 2015), https://www.newscientist.com/article/mg22630164-300-baby-genes-to-be-mapped-at-birth-in-medical-first/ [https://perma.cc/PF38-TCAS] (describing the BabySeq project, which aims to study newborn genome-wide sequencing as part of an NIH grant to Brigham & Women’s hospital). ↑
- .See John Reynolds, DNA Testing at Birth Will Be Available for Everyone, The Times (Nov. 6, 2019), https://www.thetimes.co.uk/article/dna-testing-at-birth-will-be-available-for-everyone-wbmn0zjwz [https://perma.cc/F53G-TN4D] (discussing the anticipated rollout of a newborn sequencing program that aimed to lay the groundwork for nationwide availability of screening); Clare Wilson, Sequencing the Genome of Every UK Baby Would Be an Ethical Minefield, New Scientist (Nov. 7, 2019), https://www.newscientist.com/article/2222754-sequencing-the-genome-of-every-uk-baby-would-be-an-ethical-minefield/ [https://perma.cc/P7HA-TD4J] (discussing potential ethical issues raised by nationwide newborn genome-wide sequencing). ↑
- .See, e.g., Josephine Johnston, John D. Lantos, Aaron Goldenberg, Flavia Chen, Erik Parens, Barbara A. Koenig & NSIGT Ethics and Policy Advisory Board, Sequencing Newborns: A Call for Nuanced Use of Genomic Technologies, Hastings Ctr. Rep., July–Aug. 2018, at S6, https://onlinelibrary.wiley.com/doi/full/10.1002/hast.874 [https://perma.cc/TRJ8-AX65] (“Whole-exome or whole-genome sequencing should not be used as a sole screen in state-sponsored newborn screening programs.”). ↑
- .See Nat’l Insts. of Health, NOT-OD-15-103: Enhancing Reproducibility through Rigor and Transparency (2015), https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-103.html [https://perma.cc/C576-QDZ4] (requiring “authentication of key biological and/or chemical resources”). ↑
- .See Smith, supra note 19, at 438–39 (describing that current cell line authentication standards utilize some of the same genetic markers as law enforcement and introducing an alternative marker set that does not overlap with those used for law enforcement). ↑
- .Natalie Ram, Fortuity and Forensic Familial Identification, 63 Stan. L. Rev. 751, 760 (2011) [hereinafter Ram, Fortuity and Forensic Familial Identification]. ↑
- .See 34 U.S.C. § 12592 (establishing a system for agencies to access DNA databases). ↑
- .Ram, Fortuity and Forensic Familial Identification, supra note 89, at 760; Ram, Genetic Privacy, supra note 11, at 1375. ↑
- .See CODIS-NDIS Statistics, Fed. Bureau of Investigation, https://www.fbi.gov/services/laboratory/biometric-analysis/codis/ndis-statistics [https://perma.cc/V6QF-DHFK] (last updated Oct. 2021) (showing statistics for “all [fifty] states and Puerto Rico, as well as for the . . . District of Columbia”). ↑
- .See Ram, DNA by the Entirety, supra note 81, at 903 (explaining how “[t]he immutable nature of genetic information . . . complicates individuals’ interests in their identifiable genetic information”). Significantly, as explained infra notes 30608 and accompanying text, an individual’s genetic information is also nearly always involuntarily, as well as immutably, shared, as nearly all genetic relationships are “thrust upon us, rather than voluntarily undertaken.” Ram, Investigative Genetic Genealogy, supra note 13, at 20; see also generally Ram, DNA by the Entirety, supra note 81 (exploring the implications of shared genetic data for law enforcement and other purposes). ↑
- .Indeed, researchers have recently confirmed that not even identical twins are wholly genetically similar. See Catherine Offord, Identical Twins Accumulate Genetic Differences in the Womb, The Scientist (Jan. 7, 2021), https://www.the-scientist.com/news-opinion/identical-twins-accumulate-genetic-differences-in-the-womb-68324 [https://perma.cc/44JZ-DFUQ] (“[A]t birth, so-called identical twins may already differ genetically from each other.”). ↑
- .Ram, Fortuity and Forensic Familial Identification, supra note 89, at 757. ↑
- .Ram, Genetic Privacy, supra note 11, at 1377. ↑
- .Ram, DNA by the Entirety, supra note 81, at 903; Tania Simoncelli, Dangerous Excursions: The Case Against Expanding Forensic DNA Databases to Innocent Persons, 34 J.L. Med. & Ethics 390, 390 (2006). ↑
- .Ram, Genetic Privacy, supra note 11, at 1377; see also Convicted Offenders Required to Submit DNA Samples, Nat’l Conf. State Legislatures (2013), http://www.ncsl.org/Documents/cj/ConvictedOffendersDNALaws.pdf [https://perma.cc/BP4L-4NA3] (“Forty eight states require the collection of DNA for any felony conviction and [forty two] states require the collection of samples for at least some misdemeanor convictions.”). ↑
- .Ram, Genetic Privacy, supra note 11, at 1377; DNA Arrestee Laws, Nat’l Conf. State Legislatures (2018), http://www.ncsl.org/Documents/cj/ArresteeDNALaws.pdf [https://perma.cc/2D9A-BEYP]. ↑
- .CODIS-NDIS Statistics, supra note 92. ↑
- .Ram, Genetic Privacy, supra note 11, at 1377; see also Ram, Investigative Genetic Genealogy, supra note 13, at 19 (“[A] de facto universal DNA database for Americans [is] something that no jurisdiction has indicated would be appropriate.”). In January 2020, the federal government initiated a process to collect and store genetic profiles from individuals in immigration custody in CODIS. Daniel I. Morales, Natalie Ram & Jessica L. Roberts, DNA Collection at the Border Threatens the Privacy of All Americans, N.Y. Times (Jan. 23, 2020), https://www.nytimes.com/2020/01/23/opinion/dna-collection-border-privacy.html [https://perma.cc/C6AH-937P]. This administrative rule was finalized in March 2020. DNA-Sample Collection from Immigration Detainees, 85 Fed. Reg. 13,483 (Mar. 9, 2020) (to be codified at 28 C.F.R. pt. 28). These collection efforts take CODIS beyond the scope of individuals arrested or convicted of a criminal offense, as many immigration infractions are civil in nature. Morales, Ram, & Roberts, supra. But even this expansion preserves some relationship between CODIS inclusion and government custody. ↑
- .Ram, Genetic Privacy, supra note 11, at 1377 n.112. ↑
- .See 34 U.S.C. § 12592(a)(1)(C) (“DNA samples that are voluntarily submitted solely for elimination purposes shall not be included in the National DNA Index System . . . .”). ↑
- .Ram, Genetic Privacy, supra note 11, at 1359. ↑
- .Id.; see also St. John, supra note 20 (revealing that the Golden State Killer investigation exploited more consumer-genetics databases than simply the GEDmatch database first disclosed by investigators). ↑
- .Ram, Genetic Privacy, supra note 11, at 1359; St. John, supra note 20. ↑
- .Ram, Genetic Privacy, supra note 11, at 1359. ↑
- .See, e.g., U.S. Dep’t of Just., Interim Policy: Forensic Genetic Genealogical DNA Analysis and Searching (2019), https://www.justice.gov/olp/page/file/1204386/download [https://perma.cc/U9N6-9ABD] (characterizing this method as “forensic genetic genealogical DNA analysis and searching”); Sacramento Cty. Dist. Att’y’s Off., Memorandum of Understanding: Investigative Genetic Genealogy Searching, https://chia187.wildapricot.org/resources/Documents/Sacramento%20County%20District%20Attorney%27s%20Office%20-%20IGG%20MOU%20Example.pdf [https://perma.cc/8N2C-D85U] (characterizing this method as “investigative genetic genealogy searching”). As in other work, this Article describes this method as “investigative genetic genealogy” or “IGG.” See Ram, Investigative Genetic Genealogy, supra note 13, at 19. ↑
- .See supra notes 97–101 and accompanying text. ↑
- .Ram, Investigative Genetic Genealogy, supra note 13, at 19. ↑
- .See Natalie Ram, Genetic Genealogy and the Problem of Familial Forensic Identification, in Consumer Genetic Technologies: Ethical and Legal Considerations 213 (I. Glenn Cohen, Nita Farahany, Henry T. Greely & Carmel Schachar eds., 2021) (“Proponents of using consumer genetic platforms to investigate and solve crimes have argued that the genetic data on which those investigations rely has been voluntarily uploaded and shared.”). ↑
- .Ram, Investigative Genetic Genealogy, supra note 13, at 19. As I have observed elsewhere, it is a misnomer to describe noncoding DNA as “junk” or to believe that it is uninformative as anything other than an individual identifier. Ram, Genetic Privacy, supra note 11, at 1379; Ram, DNA by the Entirety, supra note 81, at 881. But CODIS genetic profiles nonetheless utilize less, and less intentionally sensitive, genetic data than consumer-genetics platforms. ↑
- .See Ram, Genetic Privacy, supra note 11, at 1378–80 (explaining that DNA profiling companies examine on the order of 600,000 individual DNA data points). ↑
- .Id. at 1379; see also Snapshot Kinship Inference, Parabon NanoLabs, https://snapshot.parabon-nanolabs.com/#kinship [https://perma.cc/7KQA-KC3B] (advertising, by one of the leading sellers of investigative genetic genealogy services to law enforcement, that its “Snapshot” kinship analysis can “detect relatedness out to 9th-degree relatives (fourth cousins)”). ↑
- .See Ram, Genetic Privacy, supra note 11, at 1379–80 (describing the use of coding DNA data in consumer-genetics profiles). ↑
- .See Ram, Fortuity and Forensic Familial Identification, supra note 89, at 76062 (explaining the use of partial matches in CODIS and identifying relevant state policies governing such use). ↑
- .Ram, Genetic Privacy, supra note 11, at 1379. ↑
- .See Yaniv Erlich, Tal Shor, Itsik Pe’er & Shai Carmi, Identity Inference of Genomic Data Using Long-Range Familial Searches, 362 Science 690, 692 (2018) (“[W]e predict that with a database size of ~3 million U.S. individuals of European descent[,] . . . more than 99% . . . would have at least a single third-cousin match and more than 65% are expected to have at least one second-cousin match.”). ↑
- .Parabon Tops 200 Solved Cases, supra note 13. ↑
- .See Lindsey Van Ness, DNA Databases Are Boon to Police but Menace to Privacy, Critics Say, Stateline (Feb. 20, 2020), https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2020/02/20/dna-databases-are-boon-to-police-but-menace-to-privacy-critics-say [https://perma.cc/A9UP-T4G4] (reporting that Parabon “charges law enforcement $1,500 to process DNA and another $3,500 for the genealogy research time”). ↑
- .See Sarah Zhang, The Messy Consequences of the Golden State Killer Case, The Atlantic (Oct. 1, 2019), https://www.theatlantic.com/science/archive/2019/10/genetic-genealogy-dna-database-criminal-investigations/599005/ [https://perma.cc/3SU5-EBZ9] (reporting that FamilyTreeDNA, a consumer-genetics platform, “raised its price for law enforcement, from $100 to $700 per DNA-profile upload”). ↑
- .See Antonio Regalado & Brian Alexander, The Citizen Scientist Who Finds Killers from Her Couch, MIT Tech. Rev. (June 22, 2018), https://www.technologyreview.com/2018/06/22/142148/the-citizen-scientist-who-finds-killers-from-her-couch/ [https://perma.cc/MCZ5-UYFL] (profiling CeCe Moore, the most prominent genealogist involved in investigative genetic genealogy, and describing her expertise and skills). ↑
- .See Regulation of Genetic Tests, Nat’l Hum. Genome Rsch. Inst., https://www.genome.gov/about-genomics/policy-issues/Regulation-of-Genetic-Tests [https://perma.cc/X8XF-RSRF] (last updated Feb. 2, 2022) (“[M]ost genetic tests today are not regulated, meaning that they go to market without any independent analysis to verify the claims of the seller.”). ↑
- .See, e.g., Regalado & Alexander, supra note 122 (“Moore has no scientific degree. Like other prominent figures in the genealogy community, she is self-taught.”); George Woolston, South Jersey Genealogy Expert Now Helping Police Solve Crimes, Burlington Cnty. Times (Dec. 1, 2020, 4:41 PM), https://www.burlingtoncountytimes.com/story/news/2020/12/01/burlington-county-genealogy-expert-police-solve-dna-cold-cases-crimes/6476483002/ [https://perma.cc/HG5K-TP2K] (interviewing a nineteen-year-old genetic genealogist who “decided to randomly reach out to police departments across the country” when “he had some extra time on his hands”). But see Md. Code Ann., Crim. Proc. § 17-104 (West, 2021) (requiring licensing for both laboratories and genealogists involved in investigative genetic genealogy in conjunction with Maryland law enforcement). ↑
- .But see Antonio Regalado, Is the Consumer Genetics Fad Over?, MIT Tech. Rev. (Jan. 23, 2020), https://www.technologyreview.com/2020/01/23/276092/is-the-consumer-genetics-fad-over/ [https://perma.cc/5C9K-CEHD] (reporting that sales of consumer-genetics test kits “slowed dramatically in 2019”). ↑
- .See Lewis, supra note 8, at 708 (“The commercial possibilities associated with the retention and use of [dried blood spots] or access by third parties, such as the military or law enforcement, should be addressed.”); Suter, supra note 7, at 75556 n.133 (observing that “[s]ome have called for universal DNA databanking for criminal forensic purposes,” and that newborn screening “blood spots would offer an easy way to achieve this goal”). ↑
- .See James W. Hazel, Ellen Wright Clayton, B.A. Malin & Christopher Slobogin, Is It Time for a Universal Genetic Forensic Database?, 362 Sci. 898, 900 (2018) (discussing newborn screening as part of implementing a “universal” DNA database for crime-detection purposes). ↑
- .See, e.g., Maryland v. King, 569 U.S. 435, 46465 (2013) (holding that “the processing of respondent’s DNA sample’s 13 CODIS loci did not intrude on respondent’s privacy in a way that would make his DNA identification unconstitutional” because “the CODIS loci come from noncoding parts of the DNA that do not reveal the genetic traits of the arrestee,” and observing that, “[i]f in the future police analyze samples to determine, for instance, an arrestee’s predisposition for a particular disease or other hereditary factors not relevant to identity, that case would present additional privacy concerns not present here”). ↑
- .See supra notes 109–18 and accompanying text. ↑
- .See NSQAP: Newborn Screening Quality Assurance Program, Ctrs. for Disease Control & Prevention (July 27, 2020), https://www.cdc.gov/labstandards/nsqap.html [https://perma.cc/8NP5-HLC7] (describing the newborn screening quality assurance program, through which the CDC partners with all state laboratories engaged in newborn screening to perform proficiency and other quality assurance testing). ↑
- .See supra notes 5456 and accompanying text. ↑
- .See supra notes 73–79 and accompanying text. ↑
- .See supra notes 73–86 and accompanying text. ↑
- .See, e.g., Smith, supra note 19 (describing existing overlap between cell line authentication standards and CODIS loci); Watts, supra note 10 (explaining that researchers may “extract or sequence the DNA” of newborn screening samples). ↑
- .See Hazel, supra note 127, at 899 (discussing newborn screening as part of implementing a “universal” DNA database for crime-detection purposes). Of course, where law enforcement access to—or use of—newborn screening samples is precluded by law, such sequencing by law enforcement entities would be impermissible. ↑
- .See Watts, CBS13 Investigates, supra note 14 (disclosing California law enforcement’s use of newborn screening samples for investigative purposes). ↑
- .2003 GAO Report, supra note 22, at 24–25. ↑
- .Id. at 24. ↑
- .Id. at 25 tbl.6. ↑
- .Id. ↑
- .See McCandless & Wright, supra note 16, at 352–53 (describing the history of newborn screening programs in the United States and the development of the recommended screening panel in the years following the 2003 GAO Report). ↑
- .Genomics Law, LawSeq, https://lawseq.umn.edu/ [https://perma.cc/3V6E-3CNM]. ↑
- .Variations of these terms were also sometimes searched, including “newborn /2 (screen! or test!).” ↑
- .See 23 R.I. Gen. Laws § 23-13-14 (2020) (codifying the “Newborn screening program” without mention of retention, subsequent use, or confidentiality); 216-20-05 R.I. Code R. § 1.3 (2021) (promulgating regulations for the “Genetic, Metabolic, Endocrine, and Hemoglobinopathy Screening Program” without mention of retention, subsequent use, or confidentiality). ↑
- .Ky. Rev. Stat. Ann. § 214.155(1)(b) (West 2022). ↑
- .See generally 902 Ky. Admin. Regs. 4:030 (2022) (outlining the administration and testing requirements for the state’s newborn screening program but failing to address the retention and accessibility of newborn screening samples or related data). ↑
- .Tenn. Code Ann. § 68-5-406 (West 2021). The statutory scheme permits a newborn screening sample to be retained for longer than one year for specified purposes (not including law enforcement). Id. Even then, however, “the form containing the identifying information” must be “separated from the sample and destroyed, to ensure that the source of the sample cannot be identified.” Id. ↑
- .Mont. Admin. R. 37.57.321 (2022). ↑
- .See infra notes 239–43 and accompanying text. ↑
- .N.D. Admin. Code 33-06-16-05 (2022). ↑
- .15-1 Miss. Code R. § 1.4.8 (2021). ↑
- .S.D. Admin. R. 44:19:03:03 (2022). In general, traditionally “red” states were more likely to have no or inconclusive language governing law enforcement access to newborn blood samples or related data; but where policies were in place, they were more likely to clearly bar such access than bless it. See supra Fig. 1, Fig. 2. ↑
- .Wash. Admin. Code § 246-650-050(4) (2022). ↑
- .Haw. Code R. § 11-143-12 (2021). ↑
- .Conn. Gen. Stat. Ann. § 19a-25(a) (West 2021) (cross-referenced in Conn. Gen. Stat. Ann. § 19a-53(g) (West 2021)). The Connecticut Supreme Court has construed the scope of Conn. Gen. Stat. Ann. § 19a-25(a) to apply only to a narrow set of records. See Babcock v. Bridgeport Hospital, 742 A.2d 322, 352 (1999). In Babcock, the state supreme court held that, in light of the text of section 19a-25(a), the strong protections offered were applicable only to data specifically procured for the purpose of morbidity and mortality studies. Id. Were newborn screening staff to seek data protection under section 19a-25 directly, there might be limitations to the applicability to this statute to newborn screening data, particularly as those data become more expansive. But section 19a-25 is made applicable to newborn screening data by explicit cross-reference. Section 19a-53(g), part of the newborn screening statute, provides:Access to [newborn screening data] shall be limited to the department and persons with a valid scientific interest and qualification as determined by the commissioner, provided the department and such persons are engaged in demographic, epidemiologic or other similar studies related to health and agree, in writing, to maintain the confidentiality of such information as prescribed in this section and section 19a-25.
Conn. Gen. Stat. Ann. § 19a-53(g) (West 2021). Accordingly, section 19a-25’s strong protections should continue to apply to newborn screening data, whatever it includes. ↑
- .Iowa Admin. Code r. 641-4.3(8)(e) (2022). ↑
- .Conn. Gen. Stat. Ann. § 19a-25 (West 2021) (cross-referenced in Conn. Gen. Stat. Ann. § 19a-53(g) (West 2021)). ↑
- .410 Ill. Comp. Stat. Ann. 513/30 (West 2021). ↑
- .Id. at 513/30(b)(C). ↑
- .See, e.g., Chevron U.S.A. Inc. v. Echazabal, 536 U.S. 73, 80–81 (2002) (describing the canon of expressio unius est exclusio alterius, in which the expression of one item of a group implies the exclusion of another from that same group, and exploring its reach). ↑
- .Bearder v. Minnesota, 806 N.W.2d 766, 774 (Minn. 2011). ↑
- .See 2012 Minn. Sess. Law Serv. 7123–27 (West) (identifying 2012 amendments to Minnesota’s newborn screening statute). ↑
- .Minn. Stat. Ann. § 144.125 (West 2022). ↑
- .Id. § 144.125(5)(a). Research uses of newborn screening samples and related data are also further defined as “public health studies or research not related to newborn screening” or “public health studies or research.” Id. § 144.125(9). ↑
- .N.H. Code Admin. R. Ann. He-P 3008.11 (2022); see also N.H. Rev. Stat. Ann. § 132:10-a(III)(a) (West 2022) (“Any samples taken for newborn screening shall only be used for tests required under this section. No such samples may be used for other research or DNA testing purposes unless authorized by the parent or guardian.”); id. § 132:10-a(V) (“No whole-genome DNA sequencing shall be performed pursuant to this chapter unless the general court authorizes such sequencing by statute.”). ↑
- .Idaho Admin. Code r. 16.02.12.050(1) (2022). ↑
- .Id. at r. 16.02.12.050(2). ↑
- .12-5 Vt. Code R. § 7.6 (2021). ↑
- .Id. § 7.7. Vermont’s genetic privacy statute makes clear that newborn screening samples may not be included in CODIS or the state’s component database. See Vt. Stat. Ann. tit. 18, § 9332(c) (West 2022) (samples collected pursuant to, inter alia, the newborn screening program “shall not be utilized for any purpose in connection with the State DNA Data Bank, the State DNA Database, and CODIS unless specifically authorized by 20 V.S.A. chapter 113, subchapter 4”). Excluding samples and data from CODIS itself, however, does not necessarily imply that law enforcement will not seek to utilize those resources outside the confines of CODIS. ↑
- .15-1 Miss. Code R. § 1.4.8 (2021). ↑
- .S.D. Admin. R. 44:19:03:03 (2022). ↑
- .Colo. Rev. Stat. Ann. § 25-4-1003(2)(e) (West 2022). In another part of its code, Colorado provides that, “in the course of a criminal investigation or a criminal prosecution, and to the extent allowed under the federal or state constitution,” law enforcement personnel may obtain identifiable “information derived from genetic testing.” Id. § 10-3-1104.7(4). But this provision ought to be inapplicable to newborn screening data. Colorado adheres to the well-established rule of statutory construction that a specific statement (here, the provision in the newborn screening statute itself) controls over a general one (here, a genetic privacy statute focused on insurance discrimination). See Commonly Applied Rules of Statutory Construction, Off. of Legis. Legal Servs., Colo. Gen. Assembly, https://leg.colorado.gov/agencies/office-legislative-legal-services/commonly-applied-rules-statutory-construction [https://perma.cc/BZR3-VNYQ] (“If there’s a conflict between two statutory provisions—one of them a general statement and the other a specific statement—the court will apply the more specific statement as an exception to the general statement.”). ↑
- .28 Pa. Code § 28.5 (2022) (emphasis added). See Andrus v. Glover Const. Co., 446 U.S. 608, 61617 (1980) (“Where [the lawmaker] explicitly enumerates certain exceptions to a general prohibition, additional exceptions are not to be implied, in the absence of evidence of a contrary legislative intent.”). ↑
- .See Conn. Gen. Stat. Ann. § 19a-53(g) (West 2021) (requiring that “personally identifiable information” from newborn screening be used “solely for purposes of the birth defects screening program”); Haw. Code R. § 11-143 (2021) (“All information, . . . specific to individual newborns, shall be confidential and shall be used solely for the purposes of medical intervention, counseling, scientific research, or reporting.”). ↑
- .16-4107-9.0 Del. Admin. Code § 9.3 (2022) (emphasis added). Delaware law may arguably give broader authority to disclose genetic data for law enforcement purposes, see Del. Code Ann. tit. 16, § 1205(a) (West 2022) (limiting disclosure of the identity of an individual upon whom a genetic test has been performed, but not limiting disclosure of the genetic data itself); infra subsection II(B)(4)(c) (discussing these diverging policies for samples and data). Though this permission is itself in tension with the regulations governing the newborn screening program. See 16-4107-9.0 Del. Admin. Code § 9.2 (2022) (stating without exception that “[i]nformation may be disclosed by the Newborn Screening Program in summary forms, which do not identify individuals”). ↑
- .Md. Code Regs. 10.52.12.13 (2022) (emphasis added). ↑
- .Ga. Code Ann. § 33-54-3(b) (West 2022) (emphasis added). ↑
- .Okla. Admin. Code § 310:550-19-1(e) (2022) (emphasis added). ↑
- .See 12 Va. Admin. Code § 5-71-250(c) (2022) (providing that “[i]nformation that the Virginia Department of Health receives under this section is confidential and may only be used or disclosed” for specified purposes, none of which is related to law enforcement or criminal investigation”) (emphasis added). ↑
- .See Nev. Rev. Stat. Ann. § 629.151(1) (West 2021) (permitting nonconsensual acquisition of genetic information where that information is obtained “[b]y a federal, state, county or city law enforcement agency to establish the identity of a person or dead human body”); id. § 629.161(1)(b) (permitting nonconsensual retention of genetic information where “[n]ecessary to conduct a criminal investigation, an investigation concerning the death of a person or a criminal or juvenile proceeding”), id. § 629.171(1) (permitting nonconsensual disclosure of genetic information “[t]o conduct a criminal investigation, an investigation concerning the death of a person or a criminal or juvenile proceeding”). ↑
- .Id. § 629.171(1). ↑
- .Id. §§ 629.151(5), 629.171(7). ↑
- .N.J. Stat. Ann. § 10:5-47(a)(1) (West 2021); see also id. § 10:5-45(a)(1) (protecting generally against nonconsensual obtaining of genetic information but excluding from protection “genetic information obtained . . . [b]y a State, county, municipal or federal law enforcement agency for the purposes of establishing the identity of a person in the course of a criminal investigation or prosecution”); id. § 10:5-46(a)(1) (protecting generally against nonconsensual retention of genetic information but permitting retention where “necessary for the purposes of a criminal or death investigation or a criminal or juvenile proceeding”). ↑
- .Id. § 10:5-47(a)(8). ↑
- .Id. § 10:5-47(a)(4) (cross-referencing N.J. Stat. Ann. § 53:20-17 et seq. (West 2021)). ↑
- .Delaware’s genetic privacy statute is substantively identical to New Jersey’s. See Del. Code Ann. tit. 16, § 1205 (West 2022) (providing an exception for disclosure where disclosure is necessary for a criminal or death investigation or is made pursuant to CODIS or newborn screening requirements). In contrast to New Jersey, however, Delaware’s newborn screening regulations announce a more restrictive standard, at least for newborn screening samples. See 16-4107-9.0 Del. Admin. Code § 9.0 (2022) (requiring confidential treatment of records of newborns with a confirmed diagnosis of various disorders and that any individual or institutions requesting disclosure must submit a proposal to the Newborn Screening Program and Institutional Review Board of the Division of Public Health). ↑
- .See infra Part III. ↑
- .Iowa Admin. Code r. 641-4.3(7)(b)(3) (2021). ↑
- .Iowa’s regulations governing the newborn screening program also contain the clearest example of a prohibition on law enforcement access, with respect to specimens. See Iowa Admin. Code r. 641-4.3(8)(e) (2021) (prohibiting the release of such specimens for law enforcement purposes or to establish a database for forensic identification); supra text accompanying note 156. ↑
- .See Nat’l Fed’n of Indep. Bus. v. Sebelius, 567 U.S. 519, 562 (2012) (“[I]t is well established that if a statute has two possible meanings, one of which violates the Constitution, courts should adopt the meaning that does not do so.”); see infra subpart III(C) (arguing that warrantless law enforcement access to newborn screening resources for investigative use would be unconstitutional). ↑
- .Mich. Comp. Laws Ann. § 333.5431(7)(b) (West 2022); see also LaPorte v. Gordon, No. 20-10089, 2020 WL 1429496, at *2 (E.D. Mich. Mar. 24, 2020) (describing the “opt-in and opt-out processes (depending on an individual’s date of birth)” for informed parental consent to the subsequent research use of newborn screening samples in Michigan). ↑
- .See Mich. Comp. Laws Ann. §§ 333.5430–.5431 (West 2022) (describing no secondary uses other than research for newborn screening samples). Michigan law also permits a health professional to “offer to draw an additional blood specimen from the infant” that “can be used for future identification purposes.” Mich. Comp. Laws Ann. § 333.5431(9) (West, 2022). But the “future identification purposes” this provision might relate to identifying a missing person or crime victim, rather than the putative perpetrator of a crime. See Nanette Elster, Future Uses of Residual Newborn Blood Spots: Legal and Ethical Considerations, 45 Jurimetrics J. 179, 184 (2005) (citing this Michigan statute and explaining similar policies adopted in Australia for the identification of missing persons or crime victims where alternative means of identification are foreclosed or compromised). This conclusion is reinforced by the statute’s directive that the health professional “shall explain” to the new parent that this additional blood sample “should be kept in a safe place.” Mich. Comp. Laws Ann. § 333.5431(9) (West, 2022). That is, that the blood sample will go home with the parent, rather than be stored by the state. ↑
- .181 Neb. Admin. Code § 2-007.08 (2020). ↑
- .Id. ↑
- .S.C. Code Ann. Regs. 61-80(f)(3) (2022). ↑
- .Id. 61-80 app. C. ↑
- .See, e.g., Chevron U.S.A. Inc. v. Echazabal, 536 U.S. 73, 8081 (2002) (describing the expressio unius canon). ↑
- .See Utah Admin. Code r. 438-15-16 (2021) (identifying the retention and permissible use of residual newborn screening samples for “quality assessment activities” and “research” subject to conditions). ↑
- .Id. r. 438-15-15(7). ↑
- .Id. r. 438-15-15(5). ↑
- .Utah Code Ann. § 26-3-9 (West 2021). ↑
- .Mo. Ann. Stat. § 191.317(2) (West 2020). ↑
- .Id. § 191.317(1). ↑
- .See id. §§ 191.650191.703 (pertaining to HIV testing). ↑
- .See id. §§ 191.656–191.657 (outlining to whom the results of an HIV test may be disclosed). ↑
- .Other states similarly categorize newborn screening data, genetic data, or both as “confidential” or a “confidential medical record.” See, e.g., D.C. Code § 7-858.02 (2022); La. Stat. Ann. § 40:1081.10 (West 2021) (designating “the results of any prenatal or postnatal genetic tests” as “confidential medical information”); see also supra notes 174–79 (analyzing state provisions that invoke confidentiality in combination with restrictive language regarding further uses); infra notes 219–23 (analyzing state provisions that describe newborn screening resources as “confidential” but nonetheless appear to permit disclosure, including potentially to law enforcement). In the case of the District of Columbia and Louisiana, the statutory provisions providing for confidentiality lack further context that would inform either restriction of or permission for law enforcement use. Accordingly, these jurisdictions have been designated as inconclusive, as indicated in Figure 1. ↑
- .Cal. Code Regs. tit. 17, § 6505(f) (2022). ↑
- .Id. § 6502.1(a). ↑
- .Id. § 6502.1(a)(b). ↑
- .See Watts, supra note 10 (stating that newborn blood spots have been used in California for investigatory purposes). ↑
- .Wash. Admin. Code § 246-650-050(4)(a) (2022). ↑
- .105 Mass. Code Regs. 270.011(B)(7) (2022) (denoting that newborn screening data is disclosable to “anyone authorized to receive such information pursuant to a court order”); Or. Admin. R. 333-024-1090(2)(b) (2022) (designating newborn screening specimens disclosable “[i]f required by a court order”); Tex. Health & Safety Code Ann. § 33.018(a-1), (b)(3) (West 2021) (precluding the disclosure of “[r]eports, records, and information obtained or developed by the department” under the newborn screening program in response to a subpoena, but permitting such disclosure “as authorized by court order”). ↑
- .N.Y. Civ. Rights Law § 79-l(4)(c) (McKinney 2022). ↑
- .Wis. Stat. Ann. § 253.13(4) (West 2021). ↑
- .Id. § 146.82(2)(a)(4). ↑
- .See Jocelyn Kaiser, A Judge Said Police Can Search the DNA of 1 Million Americans Without Their Consent. What’s Next?, Sci. (Nov. 7, 2019), https://www.science.org/content/article/judge-said-police-can-search-dna-millions-americans-without-their-consent-what-s-next [https://perma.cc/KW63-7CSW] (discussing warrants obtained by police and served on GEDmatch to gain access to the full GEDmatch database for matching purposes after the site adopted a policy requiring existing users to opt in to law enforcement access for matching); Ancestry Transparency Report: January 2021, Ancestry (Feb. 9, 2021), https://www.ancestry.com/cs/transparency-h2-2020 [https://perma.cc/A6GE-83F7] (“Ancestry received two requests seeking access to Ancestry’s DNA database between July 1 and December 31, 2020. Ancestry challenged both of these requests, which were withdrawn. Ancestry has provided no data in response at the time of this publication.”). ↑
- .Wis. Stat. Ann. § 146.82(2)(a)(5) (West 2021). ↑
- .See State v. Popenhagen, 749 N.W.2d 611, 623 (2008) (identifying rules “to be used in interpreting the word ‘includes,’” including “ejusdem generis, which literally means ‘of the same kind’”). As the Court explained:Ejusdem generis applies when a general word (“motions” in the present case) is used in a statute and is either preceded or followed by specific words in a statutory enumeration (“motions to quash or limit” a subpoena in the present case). According to the rule of ejusdem generis, the general word is construed to embrace only items similar in nature to the enumerated items.
Id. ↑
- .E.g., W. Va. Code R. § 64-91-9 (2021). ↑
- .Wash. Admin. Code § 246-650-050(4)(iv) (2022). ↑
- .N.D. Admin. Code 33-06-16-05 (2022). ↑
- .W. Va. Code R. § 64-91-9(9.1.c) (2021). ↑
- .10-144-283 Me. Code R. § 12 (2021). ↑
- .See 45 C.F.R. § 164.512(f)(1)(ii) (2020) (allowing a “covered entity” to disclose confidential health information to law enforcement officials when complying with a court order, subpoena, summons, or administrative request). ↑
- .Ariz. Rev. Stat. Ann. § 12-2802 (2022). Like other statutes discussed here, Arizona’s statute promises confidentiality in broad terms, stating that “genetic testing and information derived from genetic testing are confidential and considered privileged.” Id. But the statute then carves out permission for disclosure as discussed above. Id. ↑
- .W. Nicholson Price II & I. Glenn Cohen, Privacy in the Age of Medical Big Data, 25 Nature Med. 37, 37, 39 (2019) (discussing the “fundamental problem . . . that the majority of health data is not covered by HIPAA at all”). ↑
- .Covered Entities and Business Associates, U.S. Dep’t of Health & Hum. Servs. (June 16, 2017), https://www.hhs.gov/hipaa/for-professionals/covered-entities/index.html [https://perma.cc/68WZ-2N9K] (defining “covered entities” and “business associates” within the scope of HIPAA). ↑
- .45 C.F.R. § 160.103 (2020). ↑
- .Idaho Admin. Code r. 16.02.12.050 (2022). ↑
- .See S.D. Admin. R. 44:19:03:03 (2022) (stating that specimens may only be used for screening of newborn infants pursuant to S.D. Codified Laws § 34-24-17); 12-5 Vt. Code R. § 1:7.6–.7 (2021) (mandating that blood spots only be used without parental consent for “the purpose of quality assurance and quality control for routine maintenance and function checks”); see also Vt. Stat. Ann. tit. 18, § 9332(c) (West 2022) (stating that samples may not be used “for any purpose in connection with the State DNA Data Bank, the State DNA Database, and CODIS”). ↑
- .15-001 Miss. Code R. § 1.4.8 (2021). ↑
- .Id. § 1.4.7. ↑
- .Id. Mississippi law may provide some protection for newborn screening data, but it is limited in scope. The state regulatory code requires that all “Live Births and Reportable Fetal Deaths with birth defects” be reported to and included in the state Birth Defect Registry. Id. § 3.1.1. Reportable birth defects include “genetic disorders” of the sort identified through newborn screening. The data in the Registry—likely including data of children with a positive newborn screening result, but not of children with negative screening results—is “privileged and may not be divulged or made public in a manner that discloses the identity of an individual whose medical records have been used for obtaining data under this section.” Id. § 3.1.1(6)(a)(i). ↑
- .See supra note 72 and accompanying text. ↑
- .See supra notes 74–82 and accompanying text. ↑
- .Conn. Gen. Stat. Ann. §§ 19a-25, 19a-53(g) (West 2022); Haw. Code R. § 11-143-12 (2021); Md. Code Regs. 10.52.12.13 (2022). ↑
- .See Nev. Rev. Stat. Ann. §§ 629.151, 629.161, 629.171 (West 2021) (listing law enforcement use as an exception to the consent requirement for obtaining, retaining, and disclosing genetic information); N.J. Stat. Ann. §§ 10:5-45, 10:5-46, 10:5-47 (West 2021) (same). ↑
- .Bearder v. Minnesota, 806 N.W.2d 766, 774 (Minn. 2011). ↑
- .See Iowa Admin. Code r. 641-4.3.(7)–(8) (2021) (allowing the release of confidential information related to newborn screening results to a representative of a state or federal agency for the purpose of performing a legally authorized function while prohibiting the use of a newborn screening specimen for law enforcement purposes); supra notes 156, 188–89 and accompanying text. ↑
- .N.M. Code R. § 7.30.6.13(C) (2021). ↑
- .N.M. Stat. Ann. § 24-1-20(D) (West 2022). ↑
- .16-4000-4107 Del. Admin. Code § 9.3 (2022). ↑
- .Del. Code Ann. tit. 16, § 1205 (West 2022). ↑
- .N.J. Stat. Ann. §§ 10:5-45, 10:5-46, 10:5-47 (West 2021); see also, e.g., Del. Code Ann. tit. 16, § 1205 (West 2022) (permitting genetic information to be disclosed when “necessary for the purposes of a criminal or death investigation or juvenile proceeding”); Nev. Rev. Stat. Ann. §§ 629.171 (West 2021) (permitting genetic information to be disclosed without consent “[t]o conduct a criminal investigation, an investigation concerning the death of a person or a criminal or juvenile proceeding”). ↑
- .See Ariz. Rev. Stat. Ann. § 12-2802 (2022) (“[G]enetic testing and information derived from genetic testing . . . shall be released only as authorized by state or federal law, including the health insurance portability and accountability act privacy standards . . . .”); 10-144-283 Me. Code R. § 12 (2021) (asserting that newborn screening samples and related data “become[] the property of the Department and may be used in compliance with confidentiality laws”); Nev. Rev. Stat. Ann. § 442.330 (West 2021) (permitting use of newborn screening data “as otherwise provided in” Nev. Rev. Stat. § 439.538, which in turn permits compliance with HIPAA); N.D. Admin. Code 33-06-16-05 (2022) (“Information and testing materials received or generated by the newborn screening program . . . are confidential except as provided by law or regulation.”); Wash. Admin. Code § 246-650-050 (2022) (“Dried blood spot samples and specimen information may only be released when required by state or federal law or under the following conditions: . . . [a] named person in a legally executed subpoena following review and approval of the state attorney general[, a] person to whom release is mandated by order of a court of competent jurisdiction.”); W. Va. Code R. § 64-91-9 (2021) (“Confidential information obtained while performing the required screenings may only be disclosed . . . [a]s required by law.”); see also Iowa Admin. Code r. 641-4.3.(7)(b)(3) (2021) (possibly permitting, perhaps through inadvertence, disclosure of newborn screening data to a “representative of a state or federal agency, to the extent that the information is necessary to perform a legally authorized function of that agency or the department”). ↑
- .105 Mass. Code Regs. § 270.011(B)(7) (2022) (“[T]he Newborn Blood Screening Program shall not disclose newborn screening results or any information or patient identifiers . . . except to: . . . anyone authorized to receive such information pursuant to a court order.”); N.M. Stat. Ann. § 24-1-20(D) (West 2022) (“The files and records of the department are subject to subpoena for use in any pending cause in any administrative proceeding or in any of the courts of the state, unless otherwise provided by law.”); N.Y. Civ. Rights Law § 79-l(4)(c) (McKinney 2022) (“the results of a genetic test may be disclosed to specified individuals without the consent of the subject of the test as provided in an order of a court of competent jurisdiction”); Or. Admin. R. 333-024-1090(2)(b) (2022) (“The Oregon State Public Health Laboratory shall only release specimens as follows: . . . If required by a court order.”); Wis. Stat. Ann. § 253.13 (West 2022) (permitting disclosure of newborn screening data as provided in Wis. Stat. Ann. § 146.82, which permits nonconsensual access to data “[u]nder a lawful order of a court of record”); Wyo. Stat. Ann. § 35-32-102(b)(ix) (West 2021) (“Except as otherwise prohibited by law, an individual’s genetic information may be obtained, retained, disclosed and used without informed consent for: . . . [c]omplying with an order of a court of competent jurisdiction.”). ↑
- .105 Mass. Code Regs. § 270.011 (2022). ↑
- .See N.Y. Civ. Rights Law § 79-l(4)(c) (McKinney 2022) (codifying protections for the confidentiality of general genetic tests). ↑
- .See Tex. Health & Safety Code Ann. §§ 33.0111, 33.018 (West 2021). ↑
- .Id. § 33.018(b)(3). ↑
- .Id. § 33.018(a-1). ↑
- .Id. § 33.018(f)(2). ↑
- .See, e.g., New State Ice Co. v. Liebmann, 285 U.S. 262, 311 (1932) (Brandeis, J., dissenting) (“It is one of the happy incidents of the federal system that a single courageous State may, if its citizens choose, serve as a laboratory; and try novel social and economic experiments without risk to the rest of the country.”); Heather K. Gerken, Foreword: Federalism All the Way Down, 124 Harv. L. Rev. 4, 6, (2010) (“Federalism is a system that permits minorities to rule, and we are intimately familiar with its benefits: federalism promotes choice, competition, participation, experimentation, and the diffusion of power.”); Henry M. Hart, Jr., The Relations Between State and Federal Law, 54 Colum. L. Rev. 489, 493 (1954) (“The federal system has the immense advantage of providing forty-eight separate centers for [legislative] experimentation.”); Hannah J. Wiseman & Dave Owen, Federal Laboratories of Democracy, 52 U.C. Davis L. Rev. 1119, 1121–23 (2018) (describing and critiquing the states-as-laboratories justification of federalism). ↑
- .See Emily Ramshaw, DNA Deception, Tex. Trib. (Feb. 22, 2010, 5:00 AM), https://www.texastribune.org/2010/02/22/dshs-turned-over-hundreds-of-dna-samples-to-feds/ [https://perma.cc/8G4W-26V9] (reporting on the discovery). The Texas Tribune reported that other states, including California, Florida, and Minnesota, also gave blood samples to this Armed Forces forensic identification project. Id. ↑
- .See supra notes 161–64 (describing Minnesota’s amendments), 24952 (describing Texas’s amendments). ↑
- .See supra notes 40–45 and accompanying text (describing the impact of the 2007 Newborn Screening Saves Lives Act, which yielded a federal baseline for newborn screening, even in the absence of a mandate, through a combination of federal standards and federal funds). ↑
- .Iowa Admin. Code r. 641-4.3(8)(e) (2021). ↑
- .Indeed, Iowa should follow its own example and adopt similar language governing its newborn screening data. ↑
- .Conn. Gen. Stat. Ann. § 19a-25 (West 2022) (cross-referenced in Conn. Gen. Stat. Ann. § 19a-53(g) (West 2022)). ↑
- .See Nat’l Fed. of Indep. Bus. v. Sebelius, 567 U.S. 519, 562 (2012) (“[I]t is well established that if a statute has two possible meanings, one of which violates the Constitution, courts should adopt the meaning that does not do so.”). ↑
- .See Emily Berman, Leah R. Fowler & Jessica L. Roberts, Trustworthy Surveillance? 15 (Aug. 19, 2021) (unpublished manuscript) (on file with author) (“Trust is central to the work of public health.”). ↑
- .Julie Henderson, Paul R. Ward, Emma Tonkin, Samantha B. Meyer, Heath Pillen, Dean McCullum, Barbara Toson, Trevor Webb, John Coveney & Annabelle Wilson, Developing and Maintaining Public Trust During and Post-COVID-19: Can We Apply a Model Developed for Responding to Food Scares?, 8 Frontiers Pub. Health no. 369, July 2020, at 1. ↑
- .See Roy M. Anderson & Robert M. May, Vaccination and Herd Immunity to Infectious Diseases, 318 Nature 323, 323 (1985) (discussing how “the level of herd immunity must simply be sufficient to reduce the susceptible fraction below the critical point”). ↑
- .See, e.g., Bill Reger-Nash, Adrian Bauman, Ben J. Smith, Cora L. Craig, Christian G. Abildso & Kevin M. Leyden, Organizing an Effective Community-Wide Physical Activity Campaign, ACSM Health & Fitness J., Sept.–Oct. 2011, at 21, 21–22 (describing the importance of community-wide campaigns for physical fitness public health efforts); A.S.M. Abdullah & C.G. Husten, Promotion of Smoking Cessation in Developing Countries: A Framework for Urgent Public Health Interventions, 59 Thorax 623, 624 (2004) (“In the US there is also a strong evidence base for the effectiveness of community based and population based interventions,” including “sustained mass media campaigns . . . .”). ↑
- .See Louise Cummings, The “Trust” Heuristic: Arguments from Authority in Public Health, 29 Health Commc’n 1043, 1045–46 (2014) (describing how low public trust in public health has led to stubbornly low MMR vaccination rates in the United Kingdom). ↑
- .See Carlo Di Pietrantonj, Alessandro Rivetti, Pasquale Marchione, Maria Grazia Debalini & Vittorio Demicheli, Vaccines for Measles, Mumps, Rubella, and Varicella in Children, Cochrane Database of Systematic Revs. (Apr. 20, 2020), https://doi.org/10.1002/14651858.CD004407.pub4 [https://perma.cc/4VG2-TZ2Z] (assessing evidence from high-quality medical literature on the safety and efficacy of the MMR vaccine); Vaccine History: Developments by Year, Child.’s Hosp. of Phila. (Mar. 30, 2021), https://www.chop.edu/centers-programs/vaccine-education-center/vaccine-history/developments-by-year [https://perma.cc/8W4H-EB95] (observing that the MMR combined vaccine was first introduced in 1971). ↑
- .A.J. Wakefield, S.H. Murch, A. Anthony, J. Linnell, D.M. Casson, M. Malik, M. Berelowitz, A.P. Dhillon, M.A. Thomson, P. Harvey, A. Valentine, S.E. Davies & J.A. Walker-Smith, Ileal-Lymphoid-Nodular Hyperplasia, Non-Specific Colitis, and Pervasive Developmental Disorder in Children, 351 Lancet 637, 638–39 (1998). ↑
- .Retraction—Ileal-Lymphoid-Nodular Hyperplasia, Non-Specific Colitis, and Pervasive Developmental Disorder in Children, 375 Lancet 445 (2010). ↑
- .Cummings, supra note 265, at 1045–46. ↑
- .See, e.g., Heidi J. Larson, Louis Z. Cooper, Juhani Eskola, Samuel L. Katz & Scott Ratzan, Addressing the Vaccine Confidence Gap, 378 Lancet 526, 531 (2011) (detailing the declines in MMR vaccine coverage after the publication of the Wakefield paper). ↑
- .See Vaccine Hesitancy: A Generation at Risk, 3 Lancet: Child & Adolescent Health 281, 281 (2019) (reporting that in the USA, “the percentage of children aged 19–35 months who received the MMR vaccine slightly decreased from 91.6% in 2011, to 91.5% in 2017, with very low rates of coverage reported in some communities (e.g., 60% in ultra-Orthodox Jews in the state of New York where a measles outbreak is ongoing)”). ↑
- .Cummings, supra note 265, at 1046. ↑
- .Vaccine Hesitancy, supra note 271, at 281. ↑
- .See Natalie Ram & David Gray, Mass Surveillance in the Age of COVID-19, J. Law Biosciences, May 8, 2020, at 1, 17 (observing that digital contact tracing has been “touted as a silver bullet that will free the American public from the strictures of social distancing”). ↑
- .Id. at 11. ↑
- .Id. ↑
- .Berman, supra note 261, at 32–34 (describing digital contact tracing technology). ↑
- .Id. at 36–37 (“Despite all the assurances about user privacy and data security, the public did not want to participate in digital contact tracing . . . . Almost three out of five Americans indicated that they would not use the Apple/Google API [for exposure notification].”). ↑
- .See Ram & Gray, supra note 274, at 13 (noting inherent limitations of GPS and Bluetooth technology that may affect the accuracy and efficacy of digital contact tracing). Reluctant cell phone users might also have had reasonable concerns about whether contact tracing of any kind would be effective in the absence of a robust testing infrastructure and support for individuals needing to quarantine. Id. at 12–13. ↑
- .Simon Munzert, Peter Selb, Anita Gohdes, Lukas F. Stoetzer & Will Lowe, Tracking and Promoting the Usage of a COVID-19 Contact Tracing App, 5 Nature Hum. Behav. 247, 249 fig.2 (2021). ↑
- .See Ram & Gray, supra note 274, at 15–16 (emphasizing that data collected from digital contract tracing should be “zealously guarded” and excluded from law enforcement efforts to prevent abuse of data). ↑
- .See Gian M. Volpicelli, The NHS coronavirus app could track how long you spend outside, Wired UK (Apr. 7, 2020), https://www.wired.co.uk/article/nhs-coronavirus-tracking-app [https://perma.cc/9SD5-U8M4] (reporting the NHS’s plans to “expand . . . its coronavirus contact-tracing app to enforce social distancing by warning people if they spend too much time outside”). ↑
- .See Ashley Southall, Scrutiny of Social-Distance Policing as 35 of 40 Arrested Are Black, N.Y. Times (Nov. 30, 2020), https://www.nytimes.com/2020/05/07/nyregion/nypd-social-distancing-race-coronavirus.html [https://perma.cc/9NHQ-9RUU] (scrutinizing enforcement of social-distancing rules in communities of color). ↑
- .See generally Berman, supra note 261 (using digital contact tracing as a case study in the contrasts between “coercive surveillance” and “cooperative surveillance”). ↑
- .Id. at 37 (suggesting that even privacy-protective digital contact tracing failed to gain mass acceptance and use because “it employs data collection mechanisms more readily associated with coercive surveillance and its concomitant threats to privacy and civil liberties”). As Berman and colleagues concede, “the public’s skepticism may have been warranted,” in light of recent news accounts indicating that the Google/Android exposure notification app failed “to keep its users’ data secure.” Id. at 38–39. Other digital contact tracing apps performed even worse. Id. at 39. ↑
- .See Paul Ohm, The Many Revolutions of Carpenter, 32 Harv. J.L. & Tech. 357, 383 (2019) (“Medical records contain symptoms, diagnoses, and prescriptions—information likely far more deeply revealing than location information.”). ↑
- .532 U.S. 67 (2001). ↑
- .Id. at 70, 72, 86. ↑
- .Id. at 78. ↑
- .Id. at 78 n.14. ↑
- .42 U.S.C. § 241(d). ↑
- .Id. § 241(d)(1)(C)–(D). ↑
- .Id. § 241(d)(1)(D). ↑
- .Id. § 241(d)(1)(E). ↑
- .See 34 U.S.C. § 12592(a) (authorizing the Director of the FBI to “establish an index of . . . DNA identification records of” persons arrested for or convicted of crimes and “analyses of DNA samples recovered from crime scenes,” as well as those “recovered from unidentified human remains” and “voluntarily contributed from relatives of missing persons”). ↑
- .Id. ↑
- .See Frequently Asked Questions on CODIS and NDIS, Fed. Bureau of Investigation, https://www.fbi.gov/services/laboratory/biometric-analysis/codis/codis-and-ndis-fact-sheet [https://perma.cc/6WXT-YYNF] (describing Family Reference Samples and their use). ↑
- .Id. ↑
- .The San Francisco Police Department’s use of DNA data from sexual assault victims to identify these individuals as suspects in unrelated crimes, by contrast, stands as a stark reminder of the potential costs of law enforcement’s breach of trust. See Abdollah, supra note 20 (documenting public criticism of the policy). Experts described such use as “highly unusual, unethical and shocking,” “absolutely wrong,” and “contradictory to any professional ethics.” Id. San Francisco’s police chief emphasized that police “don’t want our victims to worry.” Id. Tellingly, the city’s district attorney, who first disclosed and rejected the practice, explained, “Even if we’re talking about a more serious crime, it doesn’t justify the tremendous damage this kind of policy does to trust and cooperation.” Id. ↑
- .Newborn Screening Laboratory Bulletin, Ctrs. for Disease Control & Prevention (Feb. 21, 2014), https://www.cdc.gov/nbslabbulletin/bulletin.html [https://perma.cc/C2M9-8827]. ↑
- .See, e.g., Tom L. Beauchamp & James F. Childress, Principles of Biomedical Ethics 15–16 (8th ed. 2019) (identifying respect for autonomy, nonmaleficence, beneficence, and justice as core moral principles); James F. Childress, Ruth R. Faden, Ruth D. Gaare, Lawrence O. Gostin, Jeffrey Kahn, Richard J. Bonnie, Nancy E. Kass, Anna C. Mastroianni, Jonathan D. Moreno & Philip Nieburg, Public Health Ethics: Mapping the Terrain, 30 J.L. Med. & Ethics 170, 171–72 (2002) (identifying “respecting autonomous choices and actions” among “relevant general moral considerations” in public health ethics); D.L. Weed & R.E. McKeown, Ethics in Epidemiology and Public Health: Technical Terms, 55 J. Epidemiology & Cmty. Health 855, 856 (2001) (defining “[r]espect for persons” as a “prima facie principle in bioethics” that ”[i]mplies two distinct moral requirements: acknowledge autonomy and protect those with diminished autonomy”). ↑
- .Nat’l Comm’n for the Prot. of Hum. Subjects of Biomedical and Behav. Rsch., The Belmont Report (1979), https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf [https://perma.cc/3WNW-XQXM] [hereinafter Belmont Report]. So described, this principle has alternately been called one of respect for persons or for autonomy. See Beauchamp & Childress, supra note 301, at 13 (describing the principle as respect for autonomy). This Article retains the language of respect for persons. ↑
- .See John D. Arras, Principles and Particularity: The Role of Cases in Bioethics, 69 Ind. L.J. 983, 991 (1994) (arguing that “the ‘principlist’ version of applied ethics was able to virtually corner the methodological market in bioethics”); Beauchamp & Childress, supra note 301, at 13 (expounding principlism, which includes respect for autonomy, nonmaleficence, beneficence, and justice as core moral principles of biomedical ethics); Childress, supra note 301, at 171–72 (identifying principlist commitments as public health ethics as well). ↑
- .Childress, supra note 301, at 172. ↑
- .See Belmont Report, supra note 302 (identifying “informed consent” as the core application of “respect for persons”). ↑
- .Childress, supra note 301, at 172. ↑
- .See supra notes 46–51 and accompanying text. ↑
- .45 C.F.R. § 46.102(e)(1) (2020) (defining “human subject” to exclude secondary use of de-identified biospecimens or related data); see also 2018 Requirements FAQs: Newborn Blood Spot, Off. for Hum. Rsch. Prots. (Jan. 16, 2019), https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/2018-requirements-faqs/index.html [https://perma.cc/GDF6-5MLL] [hereinafter 2018 Requirement FAQs] (“Research with nonidentified newborn dried blood spots, similar to other research with nonidentified biospecimens, is not considered research with human subjects under both the 2018 and pre-2018 Requirements, and thus, is not subject to 45 CFR part 46.”). ↑
- .2018 Requirement FAQs, supra note 308. ↑
- .45 C.F.R. § 46.102(e)(7). ↑
- .Id. ↑
- .See Complaint at 4, Bearder v. Minnesota, No. 27-CV-09-5615, 2009 WL 4893192 (Minn. Dist. Ct. 2009) (requesting an injunction to prevent the collection, storage, use, and dissemination of genetic information without consent); Complaint at 9, Beleno v. Tex. Dep’t of State Health Servs., No. SA-09CA-0188-FB (W.D. Tex. 2009) (requesting an injunction to stop collecting blood samples and spots from newborn infants without consent). ↑
- .See supra notes 61–67 and accompanying text (describing the Beleno and Bearder lawsuits and their resolution). ↑
- .See, e.g., Birchfield v. North Dakota, 136 S. Ct. 2160, 2185 (2016) (holding that warrantless blood alcohol tests are unconstitutional as searches incident to arrest); Ram & Gray, supra note 274, at 9 (describing “special needs” doctrine); see also infra subpart III(C). ↑
- .Berman, supra note 261, at 8. ↑
- .See supra notes 172–79 (describing newborn screening resources as “confidential” and excluding law enforcement access). But see supra notes 219–23 (describing newborn screening resources as “confidential,” yet permitting law enforcement access in some circumstances). ↑
- .See Berman, supra note 261, at 36–42 (tracing the failure of digital contact tracing in the COVID-19 pandemic to the public’s conflation of public health surveillance with national security and traditional law enforcement surveillance, with concerns about the latter undermining efforts to achieve the former). ↑
- .U.S. Const. amend. IV. ↑
- .See Carpenter v. United States, 138 S. Ct. 2206, 2221 (2018) (“Although the ultimate measure of the constitutionality of a governmental search is reasonableness, our cases establish that warrantless searches are typically unreasonable where a search is undertaken by law enforcement officials to discover evidence of criminal wrongdoing.”) (internal quotation marks omitted). ↑
- .See., e.g., Birchfield v. North Dakota, 136 S. Ct. 2160, 2184–85 (2016) (concluding blood tests are a more intrusive alternative to breath tests and require a reasonable satisfactory justification for use without a warrant); Maryland v. King, 569 U.S. 435, 446 (2013) (differentiating a buccal swab from a blood draw and intrusion and determining that the former is a more gentle process and still constitutes a search); Schmerber v. California, 384 U.S. 757, 768, 772 (1966) (finding that blood tests constitute a search but that the facts of the record in this case showed no violation of the petitioner’s right under the Fourth Amendment). ↑
- .See, e.g., King, 569 U.S. at 465–66 (concluding taking and analyzing a cheek swab is a search); Ferguson v. City of Charleston, 532 U.S. 67, 84–86 (2001) (determining that a hospital that analyzes blood with the purpose of handing it over to police must obtain consent in light of Fourth Amendment protections); Skinner v. Ry. Lab. Execs. Ass’n, 489 U.S. 602, 616 (1989) (“The ensuing chemical analysis of the sample to obtain physiological data is a further invasion of the tested employee’s privacy interests.”). ↑
- .See Clayton, Currents in Contemporary Ethics, supra note 26, at 697 (“By the early 1970s, all states had established programs with centralized laboratories . . . .”); McCandless & Wright, supra note 16, at 352 (“In most cases, testing was performed by a state-run laboratory, typically housed in a department of health.”). ↑
- .See Skinner, 489 U.S. at 615–16 (concluding that search by private railroad was nonetheless subject to Fourth Amendment scrutiny where “[t]he Government has removed all legal barriers to the testing . . . and indeed has made plain not only its strong preference for testing, but also its desire to share the fruits of such intrusion”). As a coauthor and I have explained elsewhere:Among the factors relevant to [the government-agent] inquiry are whether a government agent directed, requested, or incentivized the search, whether the private actor believed at the time that she was acting under the direction or authority of a government agent, and whether a government agent had advance notice of the search and believed that the fruits of that search would accrue to the government.
Ram & Gray, supra note 274, at 5. ↑
- .See, e.g., Bd. of Educ. of Indep. Sch. Dist. No. 92 of Pottawatomie Cnty. v. Earls, 536 U.S. 822, 835, 838 (2002) (upholding a suspicionless search of students as part of a drug testing program); Ferguson v. City of Charleston, 532 U.S. 67, 78–79 (2001) (describing, and rejecting the application of, the special needs doctrine); Chandler v. Miller, 520 U.S. 305, 323 (1997) (holding that “where the risk to public safety is substantial and real, blanket suspicionless searches calibrated to the risk may rank as ‘reasonable’”); Vernonia Sch. Dist. 47J v. Acton, 515 U.S. 646, 665–66 (1995) (holding a policy that allows suspicionless drug testing of student athletes constitutional); Treas. Emps. v. Von Raab, 489 U.S. 656, 679 (1989) (holding the suspicionless testing of employees for illegal drugs permissible in certain situations); Skinner, 489 U.S. at 634 (holding that alcohol and drug tests under the Federal Railroad Administration’s regulations were reasonable). ↑
- .Ferguson, 532 U.S. at 78. ↑
- .Id. at 79; see also id. at 88 (Kennedy, J., concurring) (“The traditional warrant and probable-cause requirements are waived in our previous cases on the explicit assumption that the evidence obtained in the search is not intended to be used for law enforcement purposes.”). ↑
- .Id. at 78 & n.12; see Earls, 536 U.S. at 833 (“[T]he test results are not turned over to any law enforcement authority.”); Vernonia Sch. Dist., 515 U.S. at 658 (“[T]he results of the tests are disclosed only to a limited class of school personnel who have a need to know; and they are not turned over to law enforcement authorities or used for any internal disciplinary function.”); Skinner, 489 U.S. at 621 n.5 (observing that while the relevant regulations “might be read broadly to authorize the release of biological samples to law enforcement authorities, the record does not disclose that it was intended to be, or actually has been, so used[,]” and reserving the question of “whether routine use in criminal prosecutions of evidence obtained pursuant to the administrative scheme would give rise to an inference of pretext, or otherwise impugn the administrative nature of the [Federal Railway Administration’s] program”); Von Raab, 489 U.S. at 663 (“Customs employees who test positive for drugs and who can offer no satisfactory explanation are subject to dismissal from the Service. Test results may not, however, be turned over to any other agency, including criminal prosecutors, without the employee’s written consent.”); see also Chandler, 520 U.S. at 312 (rejecting suspicionless drug urinalysis for political candidates, even though “the candidate would control release of the test results: Should the candidate test positive, he or she could forfeit the opportunity to run for office, and in that event, nothing would be divulged to law enforcement officials”). ↑
- .Ferguson, 532 U.S. at 79–81. ↑
- .Id. at 71–73. ↑
- .Id. at 82. ↑
- .Id. at 78. ↑
- .Id. at 79. ↑
- .Id. at 86. ↑
- .See Newborn Screening 101, supra note 1 (“Newborn screening is a state public health service that reaches each of the nearly 4 million babies born in the United States each year.”). ↑
- .See supra notes 4–5 and accompanying text (describing the substantial public health and individual impact of newborn screening). ↑
- .Ferguson, 532 U.S. at 79. ↑
- .See, e.g., Kerns v. Bader, 663 F.3d 1173, 1185 (10th Cir. 2011) (explaining that Ferguson “expressly left open whether disclosure of preexisting medical records held by the hospital would also be a search implicating the Fourth Amendment”); 1 Wayne R. LaFave, Search & Seizure: A Treatise on the Fourth Amendment § 2.7(d) (6th ed. 2021) (analyzing that Ferguson “was a case where the hospital staff intentionally set out to obtain incriminating evidence from their patients for law enforcement purposes”) (internal quotation marks omitted). But see Kiel Brennan-Marquez, Fourth Amendment Fiduciaries, 84 Fordham L. Rev. 611, 628 (2015) (arguing that Ferguson should be understood as an exception to the third party doctrine grounded in the physician–patient fiduciary relationship); infra subpart III(C)(2) (contending that this argument, based on the third party doctrine, is inapplicable to newborn screening samples and related data). ↑
- .See Clayton, Currents in Contemporary Ethics, supra note 26, at 697 (“[B]y the early 1970s, all states had established programs with centralized laboratories.”); McCandless & Wright, supra note 16, at 352 (“In most cases, testing was performed by a state-run laboratory, typically housed in a department of health.”). ↑
- .See supra notes 46–47 and accompanying text (describing the general lack of parental consent for newborn screening). ↑
- .See Skinner v. Ry. Lab. Execs. Ass’n, 489 U.S. 602, 615–16 (1989) (concluding that search by private railroad was nonetheless subject to Fourth Amendment scrutiny where “[t]he Government has removed all legal barriers to the testing . . . and indeed has made plain not only its strong preference for testing, but also its desire to share the fruits of such intrusion”). ↑
- .Ferguson, 532 U.S. at 93 n.1 (Scalia, J., dissenting). ↑
- .Skinner, 489 U.S. at 621 n.5 (reserving the question of “whether routine use in criminal prosecutions of evidence obtained pursuant to the administrative scheme would give rise to an inference of pretext, or otherwise impugn the administrative nature of the . . . program”). ↑
- .As Jennifer Oliva has observed in another context, the fact that “law enforcement relied on a collaborative policy [in Ferguson] and not on the service of administrative subpoenas to collect private health information from another state actor” does not render Ferguson inapposite. Jennifer D. Oliva, Prescription-Drug Policing: The Right to Health Information Privacy Pre- and Post-Carpenter, 69 Duke L.J. 775, 812–13 (2020). ↑
- .569 U.S. 435 (2013). ↑
- .Id. at 465–66. ↑
- .Id. at 450–51. ↑
- .Id. at 463 (“The special needs cases, though in full accord with the result reached here, do not have a direct bearing on the issues presented in this case, because unlike the search of a citizen who has not been suspected of a wrong, a detainee has a reduced expectation of privacy.”). ↑
- .Id. ↑
- .Id. ↑
- .Ferguson v. City of Charleston, 532 U.S. 67, 79 (2001). ↑
- .Id. at 92 (Scalia, J., dissenting). ↑
- .Id. at 93–95. ↑
- .U.S. Const. amend. IV. ↑
- .389 U.S. 347 (1967). ↑
- .Carpenter v. United States, 138 S. Ct. 2206, 2213 (2018) (internal quotation marks omitted) (citing Katz v. United States, 389 U.S. 347, 361 (1967) (Harlan, J., concurring)). ↑
- .Id. ↑
- .See Smith v. Maryland, 442 U.S. 735, 745–46 (1979) (holding that sharing phone numbers with a phone company precluded a reasonable expectation of privacy for those phone numbers); United States v. Miller, 425 U.S. 435, 440, 443 (1976) (holding that the availability of personal information to bank personnel constituted a waiver of reasonable expectation of privacy for that information); Ram, Genetic Privacy, supra note 11, at 1369–70 (describing the genesis of the third party doctrine). ↑
- .Miller, 425 U.S. at 440. ↑
- .Smith, 442 U.S. at 745–46. ↑
- .Miller, 425 U.S. at 442; Smith, 442 U.S. at 742. ↑
- .Smith, 442 U.S. at 744–45; see also Miller, 425 U.S. at 43 (“The depositor takes the risk, in revealing his affairs to another, that the information will be conveyed by that person to the Government.”). ↑
- .Margot E. Kaminski, Response, Carpenter v. United States: Big Data Is Different, Geo. Wash. L. Rev.: On the Docket (July 2, 2018), https://www.gwlr.org/carpenter-v-united-states-big-data-is-different/ [https://perma.cc/555H-25ES] (describing this rule as a “central truism of U.S. privacy law” until Carpenter); see also William Baude & James Y. Stern, The Positive Law Model of the Fourth Amendment, 129 Harv. L. Rev. 1821, 1871 (2016) (“It is black-letter law under Katz that people don’t have any Fourth Amendment protection for information given to a third party.”). ↑
- .See supra notes 46–47 and accompanying text (describing the general lack of parental consent for newborn screening). ↑
- .138 S. Ct. 2206 (2018). ↑
- .Id. at 2223. ↑
- .Id. at 2219–20. ↑
- .Id. at 2214 (internal quotation marks omitted). ↑
- .Id. at 2217. ↑
- .Id. at 2219–20. ↑
- .Id. at 2217 (internal quotation marks omitted). ↑
- .See id. at 2220 (observing that “[c]ell phone location information is not truly ‘shared’ as one normally understands the term”). ↑
- .Id. at 2223; see also Ohm, supra note 286, at 370–78 (identifying and explaining these three factors as Carpenter’s “test”). ↑
- .Carpenter, 138 S. Ct. at 2218; see also Ram, Genetic Privacy, supra note 11, at 1373–74 (enumerating this factor). ↑
- .See Ram, Genetic Privacy, supra note 11, at 1381–86 (arguing that genetic data satisfies Carpenter’s first factor); see also Ohm, supra note 286, at 384 (“Without a doubt, a copy of an individual’s genome satisfies the deeply revealing nature factor.”) ↑
- .See supra notes 172–79 and accompanying text. ↑
- .569 U.S. 435, 464–65 (2013). ↑
- .Id. at 465 (“[O]nce respondent’s DNA was lawfully collected the STR analysis of respondent’s DNA pursuant to CODIS procedures did not amount to a significant invasion of privacy that would render the DNA identification impermissible under the Fourth Amendment.”). ↑
- .Id. at 464–65. ↑
- .Ram, Investigative Genetic Genealogy, supra note 13, at 19. ↑
- .King, 569 U.S. at 464–65. ↑
- .See supra notes 4–5 and accompanying text (describing the importance of early intervention for children who test positive for a screened condition). ↑
- .See Carpenter v. United States, 138 S. Ct. 2206, 2217 n.3 (2018) (treating seven days of location data as the relevant time period for consideration, where the government sought seven days of data, “even though Sprint produced only two days of records”). ↑
- .King, 569 U.S. at 462–63. ↑
- .Id. at 463. ↑
- .See Carpenter, 138 S. Ct. at 2223 (discussing the “deeply revealing” nature of cell-site location information and its “depth, breadth, and comprehensive reach” as factors that trigger Fourth Amendment protection). ↑
- .See Ohm, supra note 286, at 372 (“Depth refers to the detail and precision of the information stored.”). ↑
- .See King, 569 U.S. at 442 (discussing the “undisputed” and “unparalleled” usefulness of DNA identification). ↑
- .Ram, Genetic Privacy, supra note 11, at 1387. ↑
- .See Carpenter, 138 S. Ct. at 2218 (describing concerns about cell-site location information because it allows for a comprehensive tracking of movement). ↑
- .Ram, supra note 11, at 1387; see also Ohm, supra note 286, at 372–73 (describing Carpenter’s “breadth” analysis as considering how much data is collected about an individual). ↑
- .See Suter, supra note 7, at 730–31 (observing that every state has a newborn screening program, of which new parents are often unaware, and that “in many states, the dried blood spots (DBS) are retained for long periods or indefinitely”); see also Ohm, supra note 286, at 373 (“[C]omprehensive reach refers to the number of people tracked in the database.”). ↑
- .Carpenter, 138 S. Ct. at 2223. ↑
- .See Newborn Screening 101, supra note 1 (“Newborn screening is a state public health service that reaches each of the nearly 4 million babies born in the United States each year.”). ↑
- .See, e.g., Okla. Admin. Code § 310:550-5-1 (2022) (setting out requirements for “specimen collection” for both “hospital births” and “out-of-hospital” births); Tex. Health & Safety Code Ann. § 33.011 (West 2021) (“The physician attending a newborn child . . . shall cause the child to be subjected to screening tests approved by the department for phenylketonuria, other heritable diseases, hypothyroidism, and other disorders for which screening is required by the department.”). ↑
- .See Ohm, supra note 286, at 376 (“Some forms of data collection are inescapable because they relate to services one needs to use to be a functioning member of today’s society.”). ↑
- .See Carpenter, 138 S. Ct. at 2220 (“[C]ell phones and the services they provide are such a pervasive and insistent part of daily life that carrying one is indispensable to participation in modern society.”) (internal quotation marks omitted). ↑
- .See Suter, supra note 7, at 747 (observing that a requirement of consent for newborn screening is rare, parents are often uninformed, and states often “provide limited information about the nature of [newborn screening] or that there is an option to opt out (when there is such an option)”); see also Ohm, supra note 286, at 377 (“[Cell-site location information] is automatically part of cell service because the records are generated whenever the service is used and there is no meaningful opportunity to opt out.”). ↑
- .See supra text accompanying note 72–78. ↑
- .Carpenter, 138 S. Ct. at 2218. ↑
- .See Erin Murphy, Inside the Cell: The Dark Side of Forensic DNA 168–88 (2015) (describing law enforcement efforts to collect and store DNA data outside the CODIS framework). ↑
- .Carpenter, 138 S. Ct. at 2214 (internal quotation marks omitted). ↑
- .Whether a warrant will issue where law enforcement seeks access to a repository or database without suspicion as to any particular individual in that set remains unsettled. See, e.g., In re Search of Info. Stored at Premises Controlled by Google, 481 F. Supp. 3d 730, 756–57 (N.D. Ill. 2020) (rejecting warrant application for geofencing data because it failed to satisfy either the probable cause or particularity requirement for a lawful warrant). But see United States v. James, No. 18-cr-216 (SRN/HB), 2018 WL 6566000 (D. Minn. Nov. 26, 2018) (denying motion to suppress cell phone location data obtained from tower dumps authorized by warrant), report and recommendation adopted, No. 18-CR-216 (SRN/HB), 2019 WL 325231 (D. Minn. Jan. 25, 2019), aff’d, 3 F.4th 1102 (8th Cir. 2021), cert. denied 142 S. Ct. 1352 (2022). ↑